Write the balanced net reaction for the following half cells:
Sn2+ / Sn
Cr2+ / Cr
Answers
The balanced net reactiion for the following half cells will be
Sn + Cr²⁺ ---> Sn²⁺ + Cr
What are Half cells ?
A half cell is one of the two electrodes of an electrochemical cell.
An electrochemical cell comprises two half cells, where every half cell contains an electrode and an electrolyte.
A salt bridge or direct contact is needed to connect two half cells.
The balanced net reactiion for the given half cells will be
Sn + Cr²⁺ ---> Sn²⁺ + Cr
Learn more about Half cell here ;
https://brainly.com/question/1313684
#SPJ1
Related Questions
For 55.0seconds, Trevor was hang gliding at a constant velocity. In that time, he moved 583 meters to the southeast, and a flock of Canada geese flew past him at 17 meters per second. What was Trevor's velocity?
Answers
The velocity of Trevor, given that for 55.0 seconds, he moved 583 meters sountheast is 10.6 m/s
How do I determine the velocity of Trevor?Velocity is simply defined as the rate of change of displacement with time. Mathemathically, it is given as:
Velocity = displacement / time
With the above formula, we can obtain the velocity of Trevor as illustrated below::
Time = 55.0 secondsDisplacement = 583 meters southeastVelocity of Trevor =?Velocity = displacement / time
Velocity = 583 meters / 55.0 seconds
Velocity = 10.6 m/s
Thus, from the calculation made above, we can conclude that the velocity of Trevor is 10.6 m/s
Learn more about velocity:
https://brainly.com/question/26392384
#SPJ1
Determine the products of the reaction between tin(ii) oxalate and lithium chloride
Answers
The reaction between tin (II) oxalate and lithium chloride is that it forms tin (II) chloride and lithium oxalate, which are the products of the reaction. The balanced chemical equation for the reaction is SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4.
Tin (II) oxalate reacts with lithium chloride to form a precipitate of tin (II) chloride and lithium oxalate. The reaction between tin (II) oxalate and lithium chloride is given below.
SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4
The balanced chemical equation for the reaction is as follows:
SnC₂O₄ + 2 LiCl → SnCl₂ + Li₂C₂O4 .
SnC₂O₄ is tin (II) oxalate, while LiCl is lithium chloride.
SnCl₂ is tin (II) chloride, while Li₂C₂O4 is lithium oxalate.The products of the reaction between tin (II) oxalate and lithium chloride are tin (II) chloride and lithium oxalate. Tin (II) chloride is a white crystalline powder that is soluble in water, whereas lithium oxalate is a white solid that is insoluble in water.The reaction between tin (II) oxalate and lithium chloride is a double displacement reaction, which is also known as a metathesis reaction. When a double displacement reaction takes place, two compounds exchange their cations and anions, resulting in the formation of two new compounds.
The reaction is a double displacement reaction or metathesis reaction where two compounds exchange their cations and anions to form two new compounds.
To know more about double displacement reaction visit:
brainly.com/question/29740109
#SPJ11
2. You have 200g or a solution that contains 30g of hydrochloric acid (HCI),
what percentage of your solution is made up of HCI acid?
Answers
Answer:
the percentage of your solution that made up of HCI acid is 15%
Explanation:
The computation of the percentage of your solution that made up of HCI acid is given below:
Given that
There is 200g or a solution that have 0 g of hydrochloric acid (HCI)
Based on the above information
The percentage is
= 30g ÷ 200g
= 15%
Hence, the percentage of your solution that made up of HCI acid is 15%
Help me asap, due today

Answers
1. The equation is not balanced. The balanced equation is \(4NH_3 + 5O_2 --- > 4NO +6 H_2O\)
2. The equation is balanced.
3. The equation is not balanced. The balanced version is \(2H_2O --- > 2H_2 + O_2\)
Balancing chemical equationsA balanced chemical equation usually has the same number of atoms of different elements in the reactants and the products, even though the forms of the atoms might have changed.
Consider the first equation: \(NH_3 + O_2 --- > NO + H_2O\)
The number of hydrogen atoms is not balanced. Thus, the equation that shows balanced atoms of different elements would be: \(4NH_3 + 5O_2 --- > 4NO +6 H_2O\)
Consider the second equation: \(N_2 + 3H_2 -- > 2NH_3\)
There are 2 atoms of nitrogen in the reactants and there are also 2 in the product. The number of hydrogen atoms is 6 in the reactants and 6 in the products. Thus, it is a balanced equation.
Consider the third equation: \(2H_2O --- > H_2 + O_2\)
There are 4 hydrogen atoms in the reactant and only 2 in the products. The balanced equation would be: \(2H_2O --- > 2H_2 + O_2\)
More on balancing chemical equations can be found here: https://brainly.com/question/28294176
#SPJ1
C4H8 +O₂ → _____ CO₂ + ______ H₂O
Answers
Answer:
look at the attachment I wrote it out for you.

Manganese (IV) perbromate please put into formula form
Answers
Answer
The formula form of Manganese (IV) perbromate is
\(Mn(BrO_4)_4\)Explanation
The formula of Manganese is Mn
The formula for perbromate is BrO₄⁻
Oxidation number of Manganese (IV) = +4, That is Manganese (IV) is Mn⁺⁴
Therefore, multiply the charge of manganese by 1 and perchlorate by 4 t
The jet stream is one of the key winds that moves air masses. in which direction does the jet stream in the united state generally move air masses?
Answers
The jet stream is one of the key winds that moves air masses at the direction from west to east it moves.
What are jet streams?Jet streams are strong winds which are flow in the form of narrow bands in the upper part of the atmosphere. On the earth these winds are flows near the altitude of the tropopause. These winds carries weather systems and blows toward the colder northern air and due to the rotation of earth, its direction is from west to east.
Hence jet streams move winds from west to east direction.
To know more about jet streams, visit the below link:
https://brainly.com/question/791542
Answer: From west to east.
Explanation:
Determine the bond type formed between Hg and F
a
Covalent
b
Simple Ionic
c
Multivalent Ionic
Answers
Answer:
Multivalent Ionic
Explanation:
The type of bond formed between the atoms of two elements can easily be deduced from the magnitude of electronegativity difference between the two bonding atoms.
A summary of electronegativity differences and corresponding types of bonds are shown below as adapted from chemlibretexts, where Δχ is the difference in electronegativity ;
ionic if Δχ ≥ 2.0
polar if 2.0 > Δχ > 0.5
nonpolar if 0.5 > Δχ
To determine the type of bond between Hg and F. Hg has an electronegativity of 2 while F has an electronegativity of 4. the difference in electronegativity (Δχ) is 4 - 2 = 2
This corresponds to a multivalent ionic bond because mercurous ion is the
Hg2^2+ ion and this leads to the formation of Hg2X2 (mercury I fluoride).
A sample of an ideal gas at 1.00 atm and a volume of 1.73 L was placed in a weighted balloon and dropped into the ocean. As the sample descended, the water pressure compressed the balloon and reduced its volume. When the pressure had increased to 70.0 atm, what was the volume of the sample
Answers
Answer:
Volume is 0.0247L
Explanation:
This question involves the use of Boyle's law which states that the volume of a given mass of gas is inversely proportional to it's pressure provided that temperature remains constant.
Mathematically,
v = k/p
K = VP
P1V1 = P2V2 = P3V3 = .........=PnVn
V = volume
p = pressure
Data;
P1 =1.0atm
V1 = 1.73L
P2 = 70atm
V2 = ?
P1V1 = P2V2
V2 = P1V1 / P2
V2 = (1.0×1.73)/70
V2 = 0.0247L
The volume of the sample is 0.0247L
Things people think bad about them self
1. I'm to fat/skinny
2.I'm to ugly
3.I don't love myslef
4.why doesn't anyone like me
5. Im gonna hurt myslef
stay strong
Answers
1 2 3 4 5 and how the question ask
a
A flying plane with a total surface area of 200 m2., air acts on the plane body with a force
of 8000000N. Find the air
pressure.
Answers
The pressure of the air is 40000N/m².
To calculate the pressure of the plane, we use the formula below.
Formula:
P = F/A................. Equation 1Where:
P = Pressure of the airF = Force on the planeA = Total surface area of the plane.From the question,
Given:
F = 8000000 NA = 200 m²Substitute these values into equation 1
P = 8000000/200P = 40000 N/m².Hence, The pressure of the air is 40000N/m².
Learn more about pressure here: https://brainly.com/question/25736513
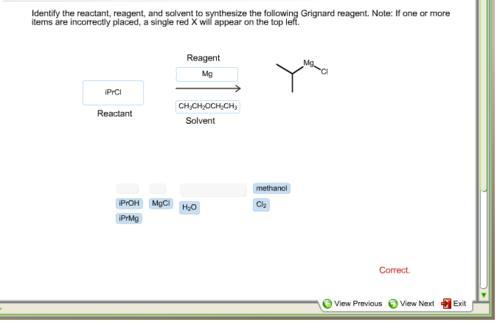
identify the reactant, reagent, and solvent used to synthesize the following alkyl lithium reagent.
Answers
reactant: iPrcl, reagent: Mg, solvent: CH3CH2OCH2CH3, used to synthesize the alkyl lithium reagent
The most prevalent carbanion reagents used in laboratory-scale synthetic chemistry are alkyllithium, alkylaluminum, and alkylmagnesium compounds; boron and silicon have very little carbanion character. The polarity of the resulting functional group is inverted since the functional carbon atom has been decreased (an originally electrophilic carbon becomes nucleophilic). Alkyl lithium and Grignard reagents are now excellent nucleophiles and helpful reactants in synthesis, as demonstrated in the modification below. Due to alkyl groups' contribution of electrons to nitrogen's more electronegative atoms, alkylamines are more basic than ammonia. Due to this inductive effect, the alkylamine nitrogen's electron density is higher than the nitrogen in ammonium.
Learn more about alkyl lithium here:
https://brainly.com/question/29341964
#SPJ4
can someone help pls

Answers
Answer:
Volume
matter
mass
Density
Acceleration
Explanation:
I had this assignment and got it right also because this is a science law and they all connect to make up a gravitational system.
What makes a charged object attract an uncharged object?
The charge in both objects move around.
The charged object is positively charged.
The charges move in the uncharged object.
The uncharged object becomes negatively charged.
Answers
Answer:
its c I think C)
The reason for this is due to the phenomenon called "Charging by Induction". What that means is that when something with a charge, for example a negative charge, is brought near an uncharged one, it induces the opposite charge onto it (positive in this case) and therefore since opposites attract, it attracts it.
The reason for this is that since, just like a positive magnet rejects and pushed away like charges, the electrons hold a negative charge, they push away any electrons that may be in the neutrally charged item causing only protons to be left, thus creating an oppositely charged object. Or vice versa.
So whatever charge the object has, it induces the opposite charge into the uncharged object, causing the charges to move only in the uncharged one.
Explanation:
which statments are true about balancing chemical reactions?
select all that apply.
a. balancing reactions should not involve trail and error
b. single atoms should be done last
c. at the end, the coefficients should be the biggest numbers possible
d. atoms that are in only one of the reactions and only one of the products should be done first.
Answers
100 points
Scientists have noted that at present the Earth is closest to the sun in January and farthest from the sun in July. The reverse will be true in 13,000 years and the Earth will then be closer to the sun in July than January. How does Earth's current proximity to the sun affect the climate in the Northern Hemisphere?
Winters are cooler and summers warmer because of the closer proximity of the Earth to the sun.
Winters are warmer and summers cooler because of the closer proximity of the Earth to the sun.
Winters are shorter and summers longer because of the closer proximity of the Earth to the sun.
Winters are longer and summers shorter because of the closer proximity of the Earth to the sun.
Answers
Winters are cooler and summers warmer because of the closer proximity of the Earth to the sun. Option A
The Earth's orbit around the sun is not a perfect circle but rather an elliptical shape. As a result, the Earth's distance from the sun varies throughout the year. The Earth is closest to the sun during a point in its orbit called perihelion, which occurs in January. Conversely, the Earth is farthest from the sun during a point called aphelion, which occurs in July.
When the Earth is closer to the sun during perihelion, the Northern Hemisphere experiences its winter season. Despite the closer proximity to the sun, the Earth's axial tilt is the primary factor that determines the seasons. During winter, the Northern Hemisphere is tilted away from the sun, resulting in shorter days and less direct sunlight. This leads to cooler temperatures during winter in the Northern Hemisphere.
In contrast, when the Earth is farther from the sun during aphelion in July, the Northern Hemisphere experiences its summer season. The Northern Hemisphere is then tilted towards the sun, resulting in longer days and more direct sunlight. This leads to warmer temperatures during summer in the Northern Hemisphere.
Therefore, option A) is the correct answer. The Earth's current proximity to the sun, with perihelion in January and aphelion in July, causes winters in the Northern Hemisphere to be cooler and summers to be warmer due to the combined effects of axial tilt and varying distance from the sun throughout the year.
Option A i9s correct.
For more such questions on Winters visit:
https://brainly.com/question/13076037
#SPJ8
Answer: It looks like none of these options are correct.
Explanation: The proximity of the Earth to the sun does not have a significant effect on the seasonal temperature changes on Earth. The tilt of Earth's axis is the primary factor that causes seasonal temperature changes.
Therefore, winters are cooler and summers are warmer because of the tilt of Earth's axis, not the proximity of the Earth to the sun.
a doctor oders 240 mg of antabuse for a pateint antuabuse comes at 0.060 g tablets (1 tablet=0.060 grams) how many tablets does the pateint need
Answers
Answer:
. 0.060 g X 240 = 14.4
Explanation: 1 tablet = 0.06 gram = 60….
A sample of Ammonia gas at 650mmHg and 15 degree has a mass of 56.8g.calculate the volume occupied by the gas. Take N=14, H=1,R=8.3145jk.
Answers
Answer:
The first thing we have to do is change and state all the units so that we can use our ideal gas law equation (\(PV = nRT\)).
650 mmHg is a pressure unit, we have to convert this to kiloPascals. We know that 760 mmHg gives us 101 kPa.
\(650 \ mmHg \ * \ \frac{101kPa}{760 mmHg} = 86 \ kPa\)
P = 86kPa
T = 15°C + 273K = 288K
R (Gas constant) = 8.31 kj/mol
Molar mass of Ammonia (\(NH_{3}\)) = (1 x 3) + (14) = 17g/mol
n (moles) = \(\frac{mass}{molar \ mass}\) \(= \frac{56.8}{17} =\) 3.34 mol
V = ?
Rearrange the equation to solve for Volume:
\(V = \frac{nRT}{P}\)
Substitute the values inside:
V = \(\frac{(3.34)(8.31)(288)}{(86)} = 93 L (rounded)\)
Therefore 93 L of volume is occupied by the ammonia gas.
Which would a chemist be most likely to study?
Answers
Answer: Chemistry, the science that deals with the properties, composition, and structure of substances (defined as elements and compounds), the transformations they undergo, and the energy that is released or absorbed during these processes.
Explanation:
I need help with this question please asap I would really appreciate it

Answers
Answer:
I think c
Explanation:
Which of the following is a property of a pure substance?
Answers
If you spilled nails in a sand box what separation tool/method could you use to clean out the sandbox so it would be safe for children to play with it's:filter,screen,magnet,Evaporation
Answers
Answer: Magnet
Explanation:Using a magnet is the best separating technique to be deployed in this case. The nails are easily picked out by just holding a magnet over the sandbox.
which of the following lewis structures would be an incomplete octet?
a. NF3
b. SO2
c. BCl3
d. CF3
E. SO3^2-
Answers
To determine which of the following Lewis structures would have an incomplete octet, we need to analyze the electron distribution for each molecule:
a. NF3 - Nitrogen (5 valence electrons) forms 3 single bonds with 3 Fluorine atoms (7 valence electrons each), completing the octet for each atom.
b. SO2 - Sulfur (6 valence electrons) forms a double bond with one Oxygen (6 valence electrons) and a single bond with another Oxygen, leaving a lone pair on the Sulfur. The octet is complete for each atom.
c. BCl3 - Boron (3 valence electrons) forms 3 single bonds with 3 Chlorine atoms (7 valence electrons each). Chlorine atoms complete their octet, but Boron only has 6 electrons around it, which makes it an incomplete octet.
d. CF3 - There is no stable molecule with this formula.
e. SO3^2- - Sulfur (6 valence electrons) forms a single bond with each of the 3 Oxygen atoms (6 valence electrons each) and has a lone pair. Each Oxygen has a formal charge of -1. The octet is complete for each atom.
So, the answer is: An incomplete octet is found in the Lewis structure of option c, BCl3.
To know more about lewis structure : https://brainly.com/question/20300458
#SPJ11
A block of concrete has a mass of 5100 g and a volume of 2500 cm3 . Calculate the density.
Answers
Answer:
2.04 g/cm3
Explanation:
Density = mass ÷ volume
= 5100 ÷ 2500
=2.04 g/cm3
Hypothesis: How easily for you think the following substances are fermented by yeast?
Answers
Yeast is a type of fungus that can ferment certain substances, meaning it breaks down sugars and converts them into alcohol and carbon dioxide. The ease with which a substance is fermented by yeast depends on a few factors, including the type of yeast being used and the composition of the substance itself.
Generally speaking, substances that contain a high amount of simple sugars are more easily fermented by yeast. This is because yeast is able to quickly and efficiently break down these sugars into alcohol and carbon dioxide. Examples of substances that are easily fermented by yeast include fruit juices, honey, and molasses.
On the other hand, substances that are more complex or contain less sugar may be more difficult for yeast to ferment. For example, yeast may have a harder time breaking down starches, such as those found in grains, without additional processing steps.
It's worth noting that different strains of yeast may also have varying levels of ability to ferment certain substances. Some strains may be better suited for fermenting certain types of beer or wine, for example, while others may be more effective at fermenting bread dough.
Overall, the ease with which a substance is fermented by yeast depends on a variety of factors, and may require some trial and error to determine the best approach for a particular substance.
Learn more about simple sugars here:
brainly.com/question/984360
#SPJ11
Which is not a form of potential energy?
gravitational
chemical
elastic
thermal
Answers
Answer:Thermal Energy is not a form of potential energy
Explanation:
\(\red{➤}\:\)\(\sf ({\frac{3}{2}})^{-1}÷ ({\frac{-2}{5}})^{-1}\)
\(\\\)
Solution:-Since the power is in negetive,we write the reciprocal of the number and then solve it like positive exponents-
\(\begin{gathered}\\\quad\longrightarrow\quad\sf( {\dfrac{3}{2}})^{-1}÷ ({\frac{-2}{5}})^{-1}\\\end{gathered} \)
\(\begin{gathered}\\\quad\longrightarrow\quad\sf ({\dfrac{2}{3}})^{1}÷ ({\dfrac{5}{-2}})^{1}\quad (a^1=a)\\\end{gathered} \)
\(\begin{gathered}\\\quad\longrightarrow\quad\sf \dfrac{2}{3}÷ \dfrac{5}{(-2)}\\\end{gathered} \)
\(\begin{gathered}\\\quad\longrightarrow\quad\sf \dfrac{2}{3}×\dfrac{(-2)}{5}\\\end{gathered} \)
\(\begin{gathered}\\\quad\longrightarrow\quad\sf \dfrac{2×(-2)}{3×5}\\\end{gathered} \)
\(\begin{gathered}\\\quad\longrightarrow\quad\boxed{\sf{ \dfrac{-4}{15}}}\\\end{gathered} \)
Know More-Laws of Exponents-
\( \sf a^m×a^n = a^{m+n} \\
\sf a^m/a^n = a^{m-n} \\
\sf{(a^m)}^n = a^{mn} \\
\sf a^n/b^n = (a/b)^n \\
\sf a^0 = 1 \\
\sf a^{-m }= 1/a^m
\)
A sample of gas has an initial volume of 12.6 L at a pressure of 1.31 atm .
Part A If the sample is compressed to a volume of 10.7 L , what is its pressure?
Answers
When the gas is compressed to a volume of 10.7 L, its pressure is approximately 1.538 atm.
To determine the final pressure of the gas when it is compressed to a volume of 10.7 L, we can use Boyle's Law. Boyle's Law states that at a constant temperature, the pressure and volume of a gas are inversely proportional.
The formula for Boyle's Law is:
P1 * V1 = P2 * V2
Where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume.
Given:
Initial volume (V1) = 12.6 L
Initial pressure (P1) = 1.31 atm
Final volume (V2) = 10.7 L (compressed volume)
We need to find the final pressure (P2).
Using Boyle's Law, we can rearrange the formula to solve for P2:
P2 = (P1 * V1) / V2
Substituting the given values:
P2 = (1.31 atm * 12.6 L) / 10.7 L
Calculating this expression:
P2 ≈ 1.538 atm
Therefore, when the gas is compressed to a volume of 10.7 L, its pressure is approximately 1.538 atm.
To learn more about Boyle's Law visit;
https://brainly.com/question/21184611
#SPJ11
The metabolic oxidation of glucose, C6H12O6, in our bodies produces CO2, which is expelled from our lungs as a gas.
C6H12O6(aq) + 6 O2(g) → 6 CO2(g) + 6 H2O(l)
Calculate the volume of dry CO2 produced at body temperature (37°C) and 0.960 atm when 24.5 g of glucose is consumed in this reaction.
Answers
Answer:
\(\large \boxed{\text{21.6 L}}\)
Explanation:
We must do the conversions
mass of C₆H₁₂O₆ ⟶ moles of C₆H₁₂O₆ ⟶ moles of CO₂ ⟶ volume of CO₂
We will need a chemical equation with masses and molar masses, so, let's gather all the information in one place.
Mᵣ: 180.16
C₆H₁₂O₆ + 6O₂ ⟶ 6CO₂ + 6H₂O
m/g: 24.5
(a) Moles of C₆H₁₂O₆
\(\text{Moles of C$_{6}$H$_{12}$O}_{6} = \text{24.5 g C$_{6}$H$_{12}$O}_{6}\times \dfrac{\text{1 mol C$_{6}$H$_{12}$O}_{6}}{\text{180.16 g C$_{6}$H$_{12}$O}_{6}}\\\\= \text{0.1360 mol C$_{6}$H$_{12}$O}_{6}\)
(b) Moles of CO₂
\(\text{Moles of CO}_{2} =\text{0.1360 mol C$_{6}$H$_{12}$O}_{6} \times \dfrac{\text{6 mol CO}_{2}}{\text{1 mol C$_{6}$H$_{12}$O}_{6}} = \text{0.8159 mol CO}_{2}\)
(c) Volume of CO₂
We can use the Ideal Gas Law.
pV = nRT
Data:
p = 0.960 atm
n = 0.8159 mol
T = 37 °C
(i) Convert the temperature to kelvins
T = (37 + 273.15) K= 310.15 K
(ii) Calculate the volume
\(\begin{array}{rcl}pV &=& nRT\\\text{0.960 atm} \times V & = & \text{0.8159 mol} \times \text{0.082 06 L}\cdot\text{atm}\cdot\text{K}^{-1}\text{mol}^{-1} \times \text{310.15 K}\\0.960V & = & \text{20.77 L}\\V & = & \textbf{21.6 L} \\\end{array}\\\text{The volume of carbon dioxide is $\large \boxed{\textbf{21.6 L}}$}\)
True or false? Metal ions and hydrogen ions both have the same type of charge.
Answers
Answer: Its true, mental ions and hydrogen ion both have the same type of charge.
Answer:
TRUE, Metal ions, and hydrogen ions both have the same type of charge. Explanation: Metal atoms lose one or more electrons at their maximum energy level and improve positively charged ions. A hydrogen ion is created when a hydrogen atom loses electrons and then becomes positively charged (charge +1).
i need to describe the organization of the periodic table
Answers
Answer:
Elements are arranged from left to right and top to bottom in order of increasing atomic number
Explanation:
This is what I remember from Chemerstry a few years ago