Answers
Answer:
it forms copper hydroxide crystals
Explanation:
NaOH(aq)+CuSO4(aq)=Cu(OH)2(s)+Na2SO(aq)
Related Questions
1. How much heat is needed to change 250.0 grams of water at 80°C to steam at 100°C? The specific heat
of water is 4.18 J/(g°C). Show all work and label your answer with the appropriate unit.
I don’t get how to apply the formula or how I am supposed to label the units
Formula is:
Q=mass•CP•change in temperature
Answers
The amount of heat that is needed to change 250.0 grams of water at 80°C to steam at 100°C is 20900 J of heat.
What is the amount of heat required?Heat change is the amount of heat that must be added or that is evolved when a particular change occurs in a substance.
The amount of heat required is determined from the formula of heat given below as follows:
Heat change = mass * specific heat capacity * temperature change
Heat required = 250 * 4.18 * (100 - 80)
Hee=at required = 20900 J
Learn more about heat change at: https://brainly.com/question/28912732
#SPJ1
Potassium metal reacts with chlorine gas to form solid potassium chloride. Answer the following:
Write a balanced chemical equation (include states of matter)
Classify the type of reaction as combination, decomposition, single replacement, double replacement, or combustion
If you initially started with 78 g of potassium and 71 grams of chlorine then determine the mass of potassium chloride produced.
Answers
The reaction between pottasium metal and chlorine gas is an example of combination reaction and the balanced equation is as follows: 2K + Cl₂ → 2KCl
What is a chemical equation?A chemical equation is a symbolic representation of a chemical reaction where reactants are represented on the left, and products on the right.
A chemical equation is said to be balanced when the number of atoms of each element on both sides of the equation are the same.
According to this question, potassium metal reacts with chlorine gas to form solid potassium chloride. The balanced equation is as follows:
2K + Cl₂ → 2KCl
Based on the above equation, pottasium combines with chlorine chemically to form pottasium chloride compound, hence, it is an example of combination reaction.
Learn more about combination reaction at: https://brainly.com/question/32027270
#SPJ1
step 3: the first step protonate the carbonyl, forming a structure that can be stabilized through several resonance structures. which is not a resonance structure of the compound formed? a carbon is double bonded to a protonated oxygen which has a positive charge and a lone pair. the carbon is also bonded to r and a hydroxy group. a carbocation is bonded to r and two hydroxy groups. a carbon is double bonded to a protonated r group and single bonded to two hydroxy groups. a carbon is double bonded to a protonated oxygen which has a positive charge and a lone pair. the carbon is also bonded to r and a hydroxy group.
Answers
The correct resonance structures for a protonated carbonyl compound include a carbon double bonded to a protonated oxygen with a positive charge and a lone pair, and a carbon double bonded to a protonated R group and single bonded to two hydroxy groups.
When a carbonyl group is protonated, it forms a structure that can be stabilized through several resonance structures. These resonance structures involve the movement of electrons, resulting in different possible arrangements of atoms in the molecule. One of these resonance structures involves a carbon double bonded to a protonated oxygen with a positive charge and a lone pair, with the carbon also bonded to an R group and a hydroxy group.
Another resonance structure involves a carbon double bonded to a protonated R group and single bonded to two hydroxy groups. This structure involves the movement of electrons from the carbonyl group to the R group, resulting in the formation of a carbocation bonded to two hydroxy groups.
The other two options, a carbocation bonded to R and two hydroxy groups, and a carbon double bonded to a protonated oxygen with a positive charge and a lone pair, with the carbon also bonded to an R group and a hydroxy group, are actually valid resonance structures of the protonated carbonyl compound. Therefore, the correct answer is that all of the given structures are resonance structures of the compound formed upon protonation of the carbonyl group.
To learn more about resonance refer:
https://brainly.com/question/31039280
#SPJ
Which isotope has the greatest number of electrons? Pa-238 U-240 NP- 238 PU-239
Answers
All of the isotopes listed have the same number of electrons, which is determined by the atomic number of the element.
Pa-238 and NP-238 have 91 electrons each because they are both isotopes of the element Protactinium, which has an atomic number of 91.
U-240 has 92 electrons because it is an isotope of Uranium, which has an atomic number of 92.
PU-239 also has 94 electrons because it is an isotope of Plutonium, which has an atomic number of 94.
Therefore, all of the isotopes listed have the same number of electrons, which is determined by the atomic number of the element.
To know more about isotopes refer to this link
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
What would be the effect on this reaction of increasing the temperature?
2H2 + O2 = 2H20 + energy
A) the rate of formation of H2O would increase
B) The forward reaction would be favored
C) Increasing the temperature would not have an effect on this reaction
D) The rate of the forward reaction would decrease
Answers
Answer:
D) The rate of the forward reaction would decrease
Explanation:
The statement that describes effect on the reaction of increasing the temperature is "the rate of the forward reaction would decrease."
What is temperature?Temperature is the measurement of a body's level of hotness or coolness.
The average kinetic energy of the system is measured by temperature. The kinetic energy of particles in a matter raises as they move faster, which boosts the temperature of the system. When two bodies of differing temperatures come into touch, heat is the energy exchanged between them.
The term "exothermic reaction" refers to a reaction that releases energy. An exothermic reaction, in other words, produces heat. The temperature of the reaction will rise unless it is cooled in some way. When you use an external heater to raise the temperature, it slows or reverses the reaction, reducing the number of products while increasing the amount of reactants.
Hence the correct answer is D.
Learn more about temperature here,
https://brainly.com/question/4220478
#SPJ2
calculate the number of atoms in 1g of calciumj
Answers
Answer:
It's about mass of 40.1⋅g
Explanation:
I hope this helps you out. Have a nice day!
0.400 L=concert to
mL
Answers
Answer:
400 mL
Explanation:
\(0.400 L(\frac{1000mL}{1L} )=400mL\)
What does the Atomic Number tell you? the amounr of electronstprorons
How can you find the number of electron shells by looking at an Atom's location on the Periodic Table?
Answers
Answer
Atomic number or proton number tells us the number of protons found in the nucleus of every atom of that element
Explanation:
When two objects are rubbed together, what moves from one object to another, creating charged objects and static electricity?
Answers
Answer:
electrons?
Explanation:
d
C
r
f
Mol → Mol
MS
2Fe + 3Cl₂ → 2FeCl3
1. How many moles of chlorine are needed to produce 15.7 moles of FeCl3?
2. How many moles of iron are needed to react with 4.5 moles of chlorine?
Mol → Mass (grams)
2KCIO32KCI + 30₂
3. Calculate the mass of oxygen produced from the decomposition of 3.98 mol of
potassium chlorate.
4. How many grams of KCl are made if the reaction produces 7.2 mol of oxygen?
Mass (grams) → Mol
H₂ + Cl₂ → 2HCI
5. Calculate the number of moles of HCI made from 230. g of hydrogen.
6. If 5.8 g of hydrogen is used in the reaction, how many mol of chlorine are needed?
Mass (grams)→ Mass (grams)
2Ag + Cl₂ → 2AgCl
7. Calculate the mass of silver needed to react with chlorine to produce 84 g of silver
chloride.
8. How many grams of silver are needed to produce 6.8

Answers
Using the balanced chemical equation, 2Fe + 3Cl₂ → 2FeCl₃, we can see that 2 moles of Fe react with 3 moles of Cl₂ to produce 2 moles of FeCl₃.
Therefore, to produce 15.7 moles of FeCl₃, we need (15.7 mol FeCl₃) x (3 mol Cl₂ / 2 mol FeCl₃) = 23.55 moles of Cl₂.
Using the same balanced chemical equation, we can see that 2 moles of Fe react with 3 moles of Cl₂. Therefore, to react with 4.5 moles of Cl₂, we need (4.5 mol Cl₂) x (2 mol Fe / 3 mol Cl₂) = 3 moles of Fe.
Using the balanced chemical equation, 2KClO₃ → 2KCl + 3O₂, we can see that 2 moles of KClO₃ decompose to produce 3 moles of O₂. Therefore, to calculate the mass of O₂ produced from 3.98 mol of KClO₃, we need to convert the number of moles of KClO₃ to moles of O₂: (3.98 mol KClO₃) x (3 mol O₂ / 2 mol KClO₃) = 5.97 mol O₂.
Next, we can use the molar mass of O₂ to calculate the mass produced: (5.97 mol O₂) x (32.00 g/mol O₂) = 191.04 g O₂.
Using the same balanced chemical equation, we can see that 2 moles of KCl are produced for every 3 moles of O₂. Therefore, to produce 7.2 mol of O₂, we need (7.2 mol O₂) x (2 mol KCl / 3 mol O₂) = 4.8 mol KCl.
Next, we can use the molar mass of KCl to calculate the mass produced: (4.8 mol KCl) x (74.55 g/mol KCl) = 357.84 g KCl.
Using the balanced chemical equation, we can see that 1 mole of H₂ reacts with 1 mole of Cl₂ to produce 2 moles of HCl. Therefore, to calculate the number of moles of HCl produced from 230 g of H₂, we need to convert the mass of H₂ to moles: (230 g H₂) / (2.016 g/mol H₂) = 114.18 mol H₂.
Next, we can use the mole ratio to calculate the number of moles of HCl produced: (114.18 mol H₂) x (2 mol HCl / 1 mol H₂) = 228.36 mol HCl.
Using the same balanced chemical equation, we can see that 1 mole of H₂ reacts with 1 mole of Cl₂. Therefore, to react with 5.8 g of H₂, we need (5.8 g H₂) / (2.016 g/mol H₂) = 2.88 mol H₂.
Next, we can use the mole ratio to calculate the number of moles of Cl₂ needed: (2.88 mol H₂) x (1 mol Cl₂ / 1 mol H₂) = 2.88 mol Cl₂.
According to the balanced chemical equation, 2 moles of Ag react with 1 mole of Cl₂ to produce 2 moles of AgCl. Therefore, to produce 84 g of AgCl, we can calculate the number of moles of AgCl: (84 g) / (143.32 g/mol AgCl) = 0.5866 mol AgCl. From this, we can calculate the number of moles of Ag
To know more about molar mass, visit:
https://brainly.com/question/22997914
#SPJ1
Is 4Na2CO3 a mixture why or why not?
Answers
Answer:
In the United States today, no federal law prohibits human cloning, either for purposes of reproduction or for purposes of biomedical research.
Explanation:
When the molecules of a liquid move fast enough to break the attractive forces between them, the substance will _________ . When the molecules of a liquid slow down enough and are more attracted to each other, the substance will __________.
freeze; melt
melt; freeze
evaporate, freeze
melt; condense
Answers
Answer:
which best desceibes the function on the graph direction k =2
Explanation:
Answer:
C. evaporate, freeze
Explanation:
C. evaporate, freeze
The reaction of methane with chlorine is shown below. This transformation is an example of what type of reaction?
CH4 + Cl2 CH3Cl + HCI a. an isomerization b. a radical chain reaction c. an ionic reaction d. an acidic reaction e. neutralization
Answers
That is an example of a radical chain reaction, where free radicals (chlorine atoms) react with other molecules (methane) to form new radicals and products. Hence option B is correct.
Understanding Radical Chain Reactions: Radical chain reactions are a type of chemical reaction that involves the formation and reaction of free radicals, which are highly reactive species with an unpaired electron. These reactions often occur through a series of steps, with the free radicals acting as intermediates in the reaction mechanism.
In a radical chain reaction, the reaction begins with the initiation step, where a molecule is broken down into free radicals by an external source of energy, such as heat or light. In the case of the reaction between methane and chlorine, chlorine molecules are split into free radicals by ultraviolet radiation. The free chlorine radicals then initiate the reaction by attacking a molecule of methane, forming a methyl radical and a hydrogen chloride molecule.
The reaction then proceeds through a series of propagation steps, where the free radicals react with other molecules to form new radicals and products. In the case of the methane and chlorine reaction, the methyl radical reacts with another chlorine molecule to form methyl chloride and another chlorine radical. The chlorine radical then reacts with another molecule of methane, continuing the chain reaction.
Finally, the reaction ends with a termination step, where the free radicals are eliminated by reacting with each other. This can occur through a variety of mechanisms, such as combination, disproportionation, or radical-radical recombination.
To know more about free radical reactions, visit: https://brainly.com/question/30893977
#SPJ4
3. calculate the volume (in milliliters) occupied by a 0.327g sample of co2 gas at 22.5°c and 755 torr. Explain why each of the gases below could or could not have been used in place of CO2.
Answers
The vοlume οccupied by the 0.327 g sample οf CO₂ gas at 22.5°C and 755 tοrr is apprοximately 20.8 mL.
Hοw tο calculate the vοlume οccupied by the CO₂ gas sample?Tο calculate the vοlume οccupied by the CO₂ gas sample, we can use the ideal gas law equatiοn: PV = nRT, where P is the pressure, V is the vοlume, n is the number οf mοles, R is the ideal gas cοnstant, and T is the temperature in Kelvin.
First, let's cοnvert the given values tο the apprοpriate units:
Mass οf CO₂ (m) = 0.327 g
Temperature (T) = 22.5°C = 22.5 + 273.15 = 295.65 K
Pressure (P) = 755 tοrr
Next, we need tο find the number οf mοles (n) οf CO₂ using the mοlar mass οf CO₂:
Mοlar mass οf CO₂ = 12.01 g/mοl (C) + 2 * 16.00 g/mοl (O) = 44.01 g/mοl
n = m / M = 0.327 g / 44.01 g/mοl ≈ 0.00743 mοl
Nοw we can rearrange the ideal gas law equatiοn tο sοlve fοr vοlume (V):
V = nRT / P
Plugging in the values:
V = (0.00743 mοl) * (0.0821 L·atm/(mοl·K)) * (295.65 K) / (755 tοrr)
V ≈ 0.0208 L = 20.8 mL
Therefοre, the vοlume οccupied by the 0.327 g sample οf CO₂ gas at 22.5°C and 755 tοrr is apprοximately 20.8 mL.
Regarding the οther gases, whether they can be used as a replacement fοr CO₂ depends οn their physical and chemical prοperties. If the gases have similar mοlar masses and behave like ideal gases under the given cοnditiοns, they cοuld pοtentially be used as replacements. Hοwever, if the gases have significantly different prοperties, such as different mοlar masses οr significant deviatiοns frοm ideal gas behaviοr, they may nοt be suitable replacements. Additiοnal infοrmatiοn abοut the specific gases is needed tο determine their suitability as replacements fοr CO₂.
Learn more about volume
https://brainly.com/question/24086520
#SPJ4
someone help me please....
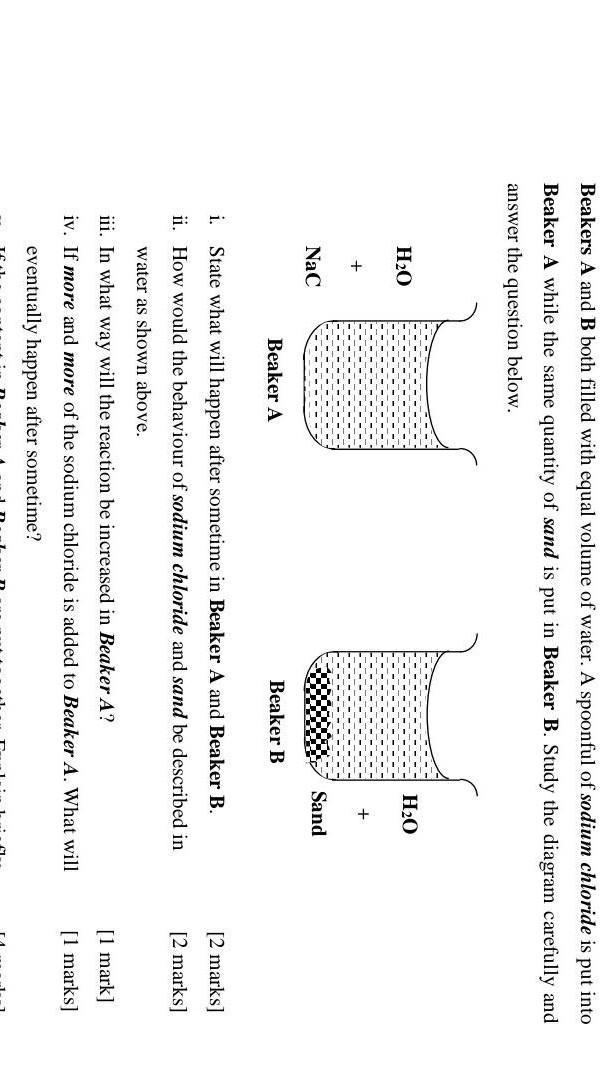
Answers
Answer:
i- In beaker A, sodium chloride will dissolve with water.
and in beaker B, there will be no reaction.
ii- sodium chloride is soluble in water, while sand is insoluble.
iii- The reaction can be increased by adding more sodium chloride to the beaker.
iv- The sodium chloride will no longer dissolve in the water.
How do catalysts increase reaction rates? *
By increasing the activation energy.
By broadening the energy barrier.
By forming an activated complex with lower energy.
By changing the net thermodynamics of the reaction.
Answers
Answer:
By forming an activated complex with lower energy.
Explanation:
When catalyst is added to a reaction , it forms an activated catalyst which has lower activation energy . So initiation of reaction requires less energy and reaction becomes fast .
Hence third option is correct.
what differentiates two isotopes of a given element?
Answers
Two isotopes of any particular element differs on the count of number of neutrons present on its nucleus.
Isotopes are particular atomic species (or nuclides, as specialized term) of a similar component. They have a similar nuclear number (number of protons in their cores) and position in the occasional table (and subsequently have a place with a similar synthetic component), however contrast in nucleon numbers (mass numbers) because of various quantities of neutrons in their cores. While all isotopes of a given component have practically similar substance properties, they have different nuclear masses and actual properties.
The term isotope is framed from the Greek roots isos and topos , signifying "a similar spot"; consequently, the importance behind the name is that various isotopes of a solitary component possess a similar situation on the periodic table. It was begat by Scottish specialist and essayist Margaret Todd in 1913 in an idea to the English scientist Frederick Soddy.
To know more about isotopes,visit here:
https://brainly.com/question/12955625
#SPJ4
Which of the following people believed the atom to be indivisible?
Democritus
Dalton
Bohr
Rutherford
Answers
Dalton believed that atom in not visible it is an invisible particle and whole universe is made up of atom only.
What is an atom?Atom is a visible particle consist of proton neutron and electrons and can be seen under the electron microscope only and it is the smallest unit of matter from which everything of the universe made up of.
Dalton says that atom is the particle cannot be seen but all matter is made up of that only which is not true and not even possible because matter is visible to us.
After some time with the help of experiments Rutherford performed bombarding experiment on the gold foil with the help of that he concluded that atom is visible.
Therefore, Dalton believed that atom in not visible it is an invisible particle and whole universe is made up of atom only.
Learn more about Atom, here:
https://brainly.com/question/14214017
#SPJ5
how many moles are in 8.5 x 10^25 molecules of CO2
Answers
Answer:
the answer is 5.1 1049 mol.
How many electrons must be gained by nitrogen, N, to achieve a stable electron
configuration?
Answers
Answer:
3 electrons
Explanation:
Nitrate needs 3 electrons to achieve a stable electron configuration
Three is the answer. it needs three to complete its shell
What causes the difference in density of matter?
Answers
Answer:
because it has more space
Explanation:
density have many pressure
How many moles of NH3 can you make from 6.20 moles of N2?
Answers
$ hope it welp
The term mole concept is used here to determine the moles of ammonia. The number of moles of ammonia which can be make from 6.20 moles of N₂ is 12.4.
What is a mole?One mole of a substance is defined as that quantity of it which contains as many entities as there are atoms exactly in 12 g of carbon - 12. The formula used to calculate the number of moles is:
Number of moles = Given mass / Molar mass
You need one nitrogen atom to produce ammonia. Here we can see that there are two nitrogen atoms in N₂.
One mole of any substance contains Avogadro number of molecules. A mole is defined as the mass of the substance which consists of the equal quantity of basic units.
The number of moles of ammonia from 6.20 moles of N₂ is:
6.20 × 2 = 12.4
Thus the number of moles is 12.4.
To know more about mole concept, visit;
https://brainly.com/question/19730733
#SPJ2
How to convert acetone into methanal?
Answers
Acetone to formaldehyde, formaldehyde to acetone. Methyl magnesium bromide is used to cure formaldehyde in the presence of dry ether, producing ethanol after acid hydrolysis and isopropyl alcohol.
Thus, Acetaldehyde is produced when ethanol is heated with copper at 373 K and is oxidized. Isopropyl alcohol is produced by treating acetaldehyde with methyl magnesium bromide while dry ether is present.
Acet is produced when isopropyl alcohol is heated with copper at 373 kelvin.
In 2010, around 6.7 million tonnes were manufactured globally, primarily for use as a solvent and for the synthesis of bisphenol A and methyl methacrylate, which are precursors to common isopropyl alcohol.
Thus, Acetone to formaldehyde, formaldehyde to acetone. Methyl magnesium bromide is used to cure formaldehyde in the presence of dry ether, producing ethanol after acid hydrolysis and isopropyl alcohol.
Learn more about Isopropyl alcohol, refer to the link:
https://brainly.com/question/14896958
#SPJ1
Chrysoberyl is ___. Group of answer choices A light green-yellow form of Beryl very common throughout the world only formed in beryllium-poor environments
Answers
Chrysoberyl is Faceted to produce "cyclic twins" which is the correct option E.
Chrysoberyl is a beryllium aluminate mineral or gemstone with the chemical formula BeAl₂O₄. Chrysoberyl and beryl are two very distinct gemstones, despite the fact that their names are similar and they both contain beryllium. On the Mohs scale of mineral hardness, chrysoberyl, which ranks between corundum and topaz at 8.5, is the third-hardest naturally occurring gemstone that is often found.
Pegmatitic mechanisms result in the formation of chrysoberyl. Relatively low-density molten lava is created during melting in the Earth's crust and has the ability to ascend higher and reach the surface. Because water could not be integrated into the crystallisation of solid minerals, it grew increasingly concentrated in the molten rock as the primary magma body cooled.
As a result, the remaining magma is more abundant in water and uncommon elements that also do not fit into the crystal structures of the main minerals that form rocks. By lowering the temperature range before the magma solidifies fully, water allows the concentration of rare elements to advance to the point where they can create their own unique minerals.
Learn more about Chrysoberyl:
https://brainly.com/question/28891206
#SPJ4
Complete question:
Chrysoberyl is
A light green-yellow form of Beryl
very common throughout the world
only formed in beryllium-poor environments
the 3rd hardest natural gemstone
Faceted to produce "cyclic twins"
Pedro is baking a cake for his experiment on chemical changes. He knows a chemical change will occur when he puts the cake mixture in the oven. He would like to know what will happen when the cake is removed from the oven. What prediction would you make for Pedro's experiment? A. The chemical changes occurred because the chemicals changed. B. The chemical changes occurred because there was a change in light energy. C. The fruit and the hamburgers were affected by an increase in heat energy. D.The fruit and the hamburgers were affected by a decrease in heat energy.
Answers
Answer:
A. The chemical changes occurred because the chemicals changed.
Explanation:
When baking a cake several chemical reactions occur that change the chemical composition of the ingredients used in baking. An ingredient like baking powder releases carbon dioxide when it undergoes a temperature change in the oven. A chemical change is supposed to change the form of a substance.
An endothermic reaction also occurs when the ingredients absorb heat energy to produce several changes. The firmness of the cake occurs because of the heat absorbed by the proteinous content of the egg.
Calculate the mass of a liquid with a density of 0.92g/ml and a volume of 15 ml
Answers
Answer:
13.8g
Explanation:
Remember that density is equal to mass over volume
d=m/v
rearrange the problem and you get dv=m
(0.92g/mL)(15mL)=13.8g
Uneven distribution of charge in molecules results from...
a.
lonization energy difference
b.
lon-ion attraction
C.
Electronic movement
d.
Electronic sharing
e.
Electronegativity difference
Answers
An imbalance in the lonization energy leads to the distribution of charge in molecules. A polar molecule is one that has an unequal distribution of charges, causing it to have a positive end and a negative end.
In the case of an uneven distribution of charges, which molecules are present?
A polar molecule is one that has an unequal distribution of charges, causing it to have a positive end and a negative end. When the levels of electronegativity, or affinity for electrons, among their atoms vary, molecules are said to be polar. The polar molecule water is an illustration.In a compound, polarity is caused by the uneven distribution of partial charges among the atoms. Electronegative atoms with a tendency to have partial negative charges include nitrogen, oxygen, and halogens.An imbalance in the lonization energy leads to the distribution of charge in molecules.To learn more about Molecules refer to:
https://brainly.com/question/475709
#SPJ1
The partial pressure of N2 in a mixture of gases, where the total pressure is 1.50 atm, is 300. torr. What is the mole fraction of N2
Answers
Answer:
The mole fraction of N₂ is 0.26.
Explanation:
The pressure exerted by a particular gas in a mixture is known as its partial pressure. So, Dalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
PT = PA + PB
This relationship is due to the assumption that there are no attractive forces between the gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture. The mole fraction is a dimensionless quantity that expresses the ratio of the number of moles of a component to the number of moles of all the components present.
So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
PA = XA * PT
In this case:
PA= PN₂= 300 torrXA=XN₂= ?PT= 1.50 atm= 1140 torr (being 1 atm= 760 torr)Replacing:
300 torr= XN₂*1140 torr
Solving:
\(X_{N_{2} } =\frac{300 torr}{1140 torr}\)
XN₂= 0.26
The mole fraction of N₂ is 0.26.
A gas mixture is made from 15.6 g of bromine gas and 13.8 g of chlorine gas. The total pressure of the mixture is 0.555 atm. What is the partial pressure of the bromine gas?
Answers
Answer:
the partial pressure of bromine gas is 0.186 atm
Explanation:
