write out proposed reactions for each combination that produced a precipitate or gas. write three forms of reaction: molecular, ionic, and net ionic for each (tip: there should be 4 total reactions, 3 forms of each, for a total of 12 equations).
Answers
To write the proposed reactions with three forms (molecular, ionic, and net ionic) for each combination that produced a precipitate or gas, a total of 12 equations are needed.
In order to provide a comprehensive answer, I would need the specific combinations of reactants involved in the experiment. If you can provide the reactants, I can write out the proposed reactions and their corresponding forms (molecular, ionic, and net ionic) for you.
The molecular equation represents the overall chemical equation with all reactants and products in their molecular forms. The ionic equation breaks down the reactants and products into their respective ions. The net ionic equation eliminates spectator ions and focuses on the actual chemical change occurring. Please provide the combinations of reactants, and I will be able to write out the proposed reactions and their forms accordingly.
Learn more about molecular: https://brainly.com/question/475709
#SPJ11
Related Questions
Which statement is not true about the periodic table?
A. Elements in the same family have the same number of protons.
B. An element of the table is represented by a box with a symbol in it.
C. Elements in the same period of the periodic table have the same
number of electron shells.
D. It is organized by atomic number.
Answers
All elements on the periodic table have a different number of protons that can be represented by its atomic number
Elements in the same family have the same number of protons. This statement is not true about the periodic table. Therefore, option A is correct.
What is periodic table ?The chemical elements are arranged in rows and columns in the periodic table, sometimes referred to as the periodic table of the elements. It is frequently used in physics, chemistry, and other sciences and is frequently regarded as a symbol of chemistry.
The table can be used by scientists to anticipate chemical reactions, study trends in the periodic properties of certain elements, and speculate on the properties of yet-to-be-discovered elements. The elements are arranged in the contemporary periodic table according to their atomic numbers and periodic characteristics.
The periodic table has changed to represent more than 150 years of chemical and physics research and development.
Thus, option A is correct.
To learn more about the periodic table, follow the link;
https://brainly.com/question/11155928
#SPJ2
Where are most volcanoes located? (Use information from the map.)
What is happening to the earth’s crust in these locations?
Answers
experiment 2: suppose you added 0.5 g of fp sample 1 instead of 2.0 g, what would happen to the freezing point temperature of the solution?
Answers
If you added 0.5 g of sample 1 instead of 2.0 g, the freezing point temperature of the solution would decrease.
When a solute is added to a solvent, it disrupts the formation of the solvent's crystal lattice structure, lowering the freezing point of the solution. The extent to which the freezing point is lowered depends on the concentration of the solute particles in the solution. In this case, by reducing the amount of sample 1 from 2.0 g to 0.5 g, the concentration of solute particles in the solution would decrease.
Since the freezing point depression is directly proportional to the concentration of solute particles, a decrease in the amount of sample 1 would result in a smaller decrease in the freezing point temperature compared to if 2.0 g were added. In other words, the solution would experience a less significant decrease in freezing point temperature with only 0.5 g of sample 1.
Learn more about: freezing point depression
brainly.com/question/30389524
#SPJ11
in the bohr model of the hydrogen atom, the electron moves in a circular orbit of radius 5.3Ãâ€"10−11m with speed 2.2Ãâ€"106m/s .
Answers
he Bohr model of the hydrogen atom states that the electron moves in a circular orbit with a radius of 5.3×10⁻¹¹m and a speed of 2.2×10⁶m/s.
In the Bohr model, electrons orbit the nucleus in specific energy levels. The radius of the orbit is determined by the energy level the electron occupies. In this case, the electron is in a specific energy level that corresponds to a circular orbit with a radius of 5.3×10⁻¹¹m. The speed of the electron in this orbit is 2.2×10⁶m/s. This means that the electron is moving at a very high speed around the nucleus.
The Bohr model helps us understand the quantized nature of electron energy levels and provides a simplified representation of the hydrogen atom. It is important to note that this model has limitations and is an approximation of the more complex behavior of electrons in atoms as described by quantum mechanics.
Learn more about Bohr model here:
https://brainly.com/question/13606024
#SPJ11
How many grams of CO2 form when 7.50 g of C2H5OH are produced?
Answers
To determine the grams of CO2 formed when 7.50 g of C2H5OH (ethanol) is produced, we need to consider the balanced chemical equation for the combustion of ethanol:
C2H5OH + 3O2 -> 2CO2 + 3H2O
From the balanced equation, we can see that for every 1 mole of C2H5OH, 2 moles of CO2 are produced. We can use the molar mass of ethanol and the molar mass of CO2 to calculate the grams of CO2 produced.
The molar mass of C2H5OH is calculated as follows:
(2 x molar mass of C) + (6 x molar mass of H) + molar mass of O
(2 x 12.01 g/mol) + (6 x 1.008 g/mol) + 16.00 g/mol = 46.07 g/mol
Now we can set up a proportion to calculate the grams of CO2:
(7.50 g C2H5OH) / (46.07 g/mol C2H5OH) = (x g CO2) / (44.01 g/mol CO2)
Cross-multiplying the proportion:
7.50 g C2H5OH * (44.01 g/mol CO2) = 46.07 g/mol C2H5OH * x g CO2
Simplifying the expression:
x = (7.50 g C2H5OH * 44.01 g/mol CO2) / 46.07 g/mol C2H5OH
Calculating the result:
x ≈ 7.17 g CO2
Therefore, approximately 7.17 grams of CO2 are formed when 7.50 grams of C2H5OH are produced.
PLS HELP!!!!!!!!!!!!!!!

Answers
Why is it important for lab safety to know the proper names of equipment in a lab
Answers
Answer:
Knowing your lab equipment and their names will aid in having a successful experiment and may help in correcting errors.
If you do not know your lab equipment, that will only result in having a lack of knowledge of the equipment or not knowing how to correct a mistake in an experiment.
Explanation:
Hope I helped.
The photoelectron spectrum for the element nitrogen is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the ato
f the atom?
A. The leftmost peak represents the valence electrons.
B. The two peaks at the right represent a total of three electrons.
C. The electrons in the ls sublevel have the smallest binding energy
D. The electrons in the 2p sublevel have the smallest binding energy
Answers
The electrons in the 2p sub level have the smallest binding energies, which is the best explanation for how the spectrum is consistent with the electron shell model of the atom. Option D is correct as a result.
Photoemission spectroscopy, often referred to as photoelectron spectroscopy, measures the amount of energy emitted by electrons from solids, gases, or liquids via the photoelectric effect. This procedure involves getting the energy for the electrons from an outside source, such as sunlight.
The photoelectric effect is a process in which electrons receive energy from an external source, such as sunlight, become excited, and transition from the ground state to the excited state. As a result of this process, there is a constant flow of electrons, which in turn causes a flow of energy.
Learn more about spectrum here:
https://brainly.com/question/11837978
#SPJ4
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
during what part of the day (night or day) can a solar be observed? explain your answer.
subject is science, please help me thx yal
Answers
Hope it helped
CH4 (g)+ 202(g)=CO2(g) + 2H2O(l)If 133.2 grams of CH4 react with excess O2 , how many grams of H2O result?
Answers
299.7g of H2O will be produced.
1st) With the balanced chemical equation and the molar mass of CH4 and H2O, we can calculate the amount of H2O that is produced by stoichiometry:
- CH4 molar mass: 16g/mol
- H2O molar mass: 18g/mol
According to the balanced equation, 16g of CH4 will produce 36g of H2O (2 x 18g).
2nd) Now, with a mathematical rule of three we can calculate the grams of water that will be produced from 133.2 grams of CH4:
\(\begin{gathered} 16gCH_4-36gH_2O \\ 133.2gCH_4-x=\frac{133.2gCH_4\cdot36gH_2O}{16gCH_4} \\ x=299.7gH_2O \end{gathered}\)Finally, 299.7g of H2O will be produced from 133.2 grams of CH4.
Select the correct answer.
A compound has a molecular weight of 112.124 atomic mass units and the empirical formula C3H4O. What is the molecular formula of the compound? Use the periodic table to help you.
A.
C6H8O
B.
C9H12O3
C.
C8H4O2
D.
C4H8O2
E.
C6H8O2
.
Answers
Answer:
E
Explanation:
The empirical formula has a mass of
3*C = 3*12 = 36
4*H = 4*1 = 4
1*O = 1 * 16 = 16 Add
Total = 56
The actual mass of the molecule in nature is 112
Multiplier = nature's mass / empirical formula mass
Values
Nature's mass = 112
Empirical mass = 56
Solution
Multiplier = Natures mass/Empirical Mass
Multiplier = 112 / 56
Multiplier = 2
Formula
Nature's formula = (Emperical Mass)_multiplier
Nature's formula = (C3H4O)_2 Multiply
Nature's formula = C6H8O2
Check
C6 = 6*C = 6*12 = 72
H8 = 8*H = 8*1 = 8
O2 = 2*O = 2*16 = 32 Add
Total 112 which checks with what is given.
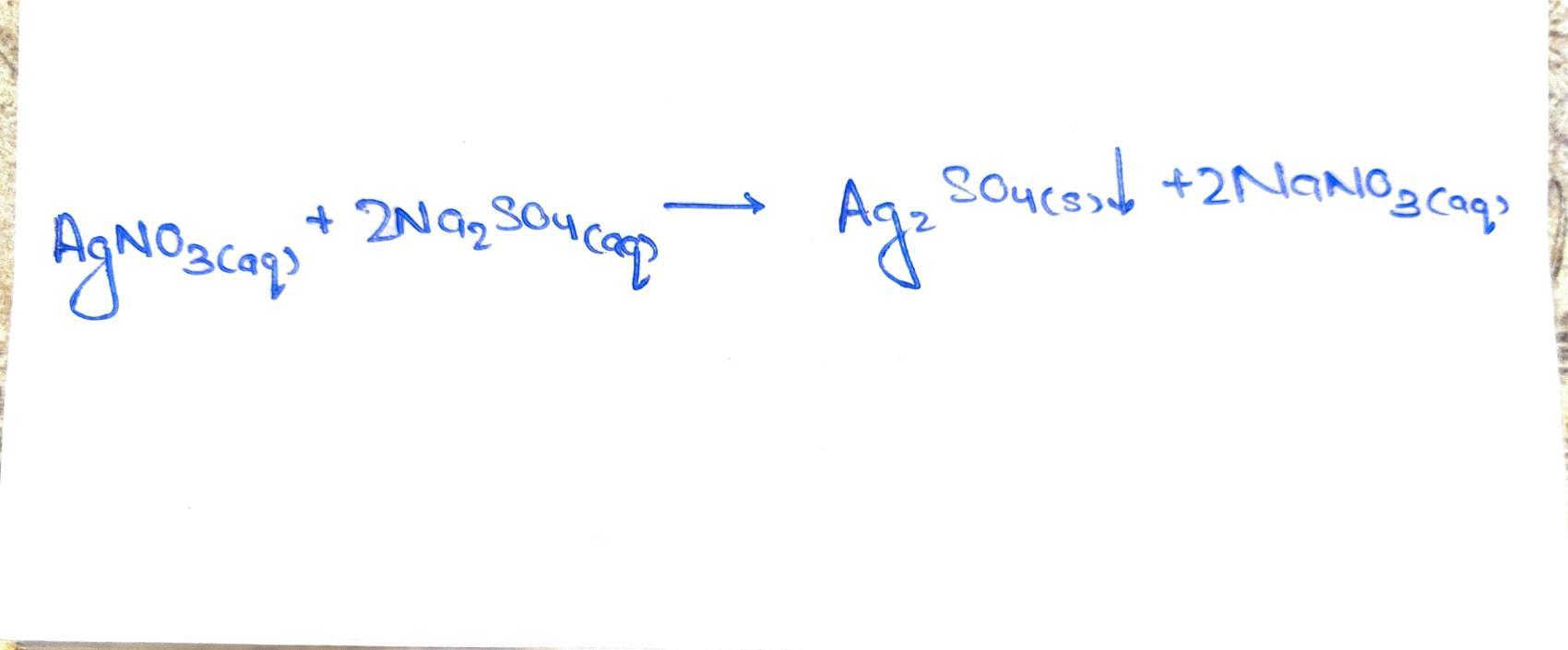
complete and balance the following equation. you must include states of matter. you do not need to write the ionic and net ionic equations. (all answers on paper) agno3 (aq) na2so4 (aq) -->
Answers
A balanced equation represents a chemical reaction, where the number of atoms of each element is equal on both sides of the equation. The balanced equation for the reaction is \(AgNO_3_{(aq)} + 2 Na_2SO_4_{(aq)\) → \(Ag_2SO_4_{(s)} + 2 NaNO_3_{(aq).\)
In this reaction, silver nitrate reacts with sodium sulfate to form silver sulfate as a solid precipitate and sodium nitrate in the aqueous form. The given equation represents a double replacement or precipitation reaction. Silver nitrate (\(AgNO_3\)) is a soluble compound in water, and sodium sulfate (\(Na_2SO_4\)) is also soluble. The balanced equation shows that two moles of sodium sulfate react with one mole of silver nitrate.
\(AgNO_3_{(aq)} + 2 Na_2SO_4_{(aq)\) → \(Ag_2SO_4_{(s)} + 2 NaNO_3_{(aq).\)
The reaction results in the formation of silver sulfate (\(Ag_2SO_4\)) as a solid precipitate, which appears as a white precipitate in the reaction mixture. Sodium nitrate (\(NaNO_3\)) is also formed, remaining in the aqueous form as it is soluble in water.
The balanced equation ensures that the number of atoms of each element is the same on both sides of the equation, satisfying the law of conservation of mass. It shows that two moles of \(AgNO_3\)react with four moles of \(Na_2SO_4\)to produce two moles of \(Ag_2SO_4\)and four moles of \(NaNO_3\).
Learn more about double replacement reaction here:
https://brainly.com/question/11725360
#SPJ11
Which of the following diagram is the correct electron dot diagram for AL

Answers
Answer:
the answer is a
Explanation:
In the box below, draw the open-chain structure (as a Fischer projection) of 2-deoxy-D-glucose. You may draw your Fischer projection without using wedged or hashed bonds. Align the Fischer projection vertically, e.g. Show explicitly the bonds to any hydrogens attached to chiral carbons. Do not show bonds to other hydrogens. A start structure for you modify is provided in the sketcher.
Answers
Below is an example of the open-chain structure (as a Fischer projection) of 2-deoxy-D-glucose.
The Fischer projection should be aligned vertically, with the carbon atoms at the top and bottom and the oxygen atoms on the left and right. The chiral carbon atoms should be labeled and any hydrogens attached to them should be explicitly shown. Note that bonds to other hydrogens should not be shown.
O O | /| | / | | H | H | H | | H | | H | | H | H | H | | H | |H | C--C--C--C--C
The diagram provided is a Fischer projection of 2-deoxy-D-glucose. The vertical alignment of the carbon atoms at the top and bottom, and the oxygen atoms on the left and right is correct. The chiral carbon atoms should be labeled and any hydrogens attached to them should be explicitly shown. Note that bonds to other hydrogens should not be shown.
Learn more about Fischer projection:
https://brainly.com/question/30088701
#SPJ4
Explain how a synthesis reaction is useful to a pharmacologist
Answers
In most drug discovery efforts, compound synthesis is regarded as the rate-limiting phase to accommodate various functional groups.
One of the most typical kinds of chemical reactions is a synthesis reaction, also known as a direct combination reaction. A + B AB is the result of the reaction between two and more chemical species in a synthesis.
The synthesis process is simple to identify in this form since there are more reactants then products. One bigger compound is created when multiple reactants come together. Synthesis reactions can be thought of as the opposite of breakdown processes. In most drug discovery efforts, compound synthesis is regarded as the rate-limiting phase to accommodate various functional groups.
To know more about synthesis reaction, here:
https://brainly.com/question/16987748
#SPJ1
Hello! can someone explain to me how to solve this?How many moles of tungsten atoms are in 4.8 * 10^25 atoms of tungsten?

Answers
How many moles of tungsten atoms are in 4.8 * 10^25 atoms of tungsten?
Avogadro's number is the number of particles that we have in one mol of particles. It is 6.022 *10^23. For example: in 1 mol of atoms there are 6.022*10^23 atoms.
It's like a dozen. When we say a dozen atoms, we mean 12 atoms. So, when we say a mol of tungsten atoms, we mean 6.022 *10^23 atoms of tungsten.
1 mol of atoms of tungsten = 6.022 *10^23 atoms of tungsten
We will use that relationship to find the number of moles of tungsten atoms that we have in 4.8 *10^25 atoms of tungsten.
number of moles of tungsten atoms = 4.8 *10^25 atoms * 1 mol of atoms/(6.022 *10^23 atoms)
number of moles of tungsten atoms = 79.7 moles = 8 *10^1 moles
number of moles of tunsten atoms = 8.10^1 moles
Answer: b) 8*10¹ moles
Could someone help me answer these questions with the answer and typed steps for how each answer was found? I asked this question previously but, I could not read the handwritten answer.
7. A 25 g soil sample was extracted with 75 mL of NH4OAc (pH 7.0), and the filtrate was analyzed
on an atomic absorption unit. The following results were obtained:
100 mg/L Ca2+, 45 mg/L Mg2+, 85.5 mg/L K+, 94.2 mg/L Al3+ and 8.0 mg/L H+.
a. What is the CEC in cmol(+)/kg for this sample?
b. What is the % B.S. for this soil?
c. What is the % acid saturation for this soil sample?
Answers
The CEC for this soil sample is 675.2 cmol(+)/kg.
The % Base Saturation for this soil sample is approximately 136.62%.
The % Acid Saturation for this soil sample is approximately 60.55%.
To calculate the CEC, % Base Saturation (B.S.), and % Acid Saturation for the given soil sample:
a. Calculation of CEC (Cation Exchange Capacity):
CEC is the sum of exchangeable cations in the soil. From the given results, we have:
CEC = Ca2+ + Mg2+ + K+ + Al3+
CEC = (100 mg/L + 45 mg/L + 85.5 mg/L + 94.2 mg/L) / (25 g / 1000)
CEC = 168.7 mg / (25 g / 1000)
CEC = 675.2 cmol(+)/kg
b. Calculation of % Base Saturation (B.S.):
% B.S. represents the percentage of CEC occupied by base cations. In this case, we consider Ca2+, Mg2+, and K+ as base cations. The formula to calculate % B.S. is:
% B.S. = (Ca2+ + Mg2+ + K+) / CEC * 100
% B.S. = (100 mg/L + 45 mg/L + 85.5 mg/L) / (168.7 cmol(+)/kg) * 100
% B.S. = 230.5 mg / (168.7 cmol(+)/kg) * 100
% B.S. = 136.62%
c. Calculation of % Acid Saturation:
% Acid Saturation represents the percentage of CEC occupied by acid cations, in this case, H+ and Al3+. The formula to calculate % Acid Saturation is:
% Acid Saturation = (H+ + Al3+) / CEC * 100
% Acid Saturation = (8.0 mg/L + 94.2 mg/L) / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 102.2 mg / (168.7 cmol(+)/kg) * 100
% Acid Saturation = 60.55%
Please note that the given values were in milligrams per liter (mg/L), and the CEC and % Saturation values were calculated assuming a conversion from mg/L to cmol(+)/kg using the mass of the soil sample (25 g).
TO know more about CEC (Cation Exchange Capacity)
https://brainly.com/question/30689981
#SPJ11
What is a scatter plot?
A. A table of measurement data used to construct a graph
B. A display of points that shows the relationship between two sets of data
C. A graph that has several different lines scattered on a coordinate plane
D. A graph that is nonlinear
PLZ, ANSWER, AND ILL MARK YOU THE BRAINLIEST!
Answers
Explanation:
A..........is the correct answer
if it helps you then plzz mark me brainliest
Answer:
The other answer is wrong! Its...
B. A display of points that shows the relationship between two sets of data
Explanation:
Hope this helps!
differentiate between edible and non edible mushroom
Answers
Edible mushrooms: Consuming edible mushrooms is safe and provides health advantages like fiber, vitamins, and minerals.
Examples: Button mushrooms.Non-Edible mushrooms: Mushrooms that cannot be eaten could be harmful or have unappealing flavors and textures that could be harmful if consumed.
Examples: Death Cap.
why are protons (h+) pumped across the inner mitochondrial membrane?
Answers
Protons (H⁺) are pumped across the inner mitochondrial membrane as part of the process called electron transport chain, which is an essential step in cellular respiration.
The electron transport chain is responsible for generating adenosine triphosphate (ATP), the main energy currency of cells.
During cellular respiration, electrons are transferred from high-energy molecules (such as glucose) through a series of electron carriers embedded in the inner mitochondrial membrane. As electrons pass through the electron transport chain, energy is released and used to pump protons (H⁺) from the mitochondrial matrix to the intermembrane space.
There are some several reasons;
Establishing an Electrochemical Gradient; The pumping of protons across the inner mitochondrial membrane creates an imbalance of protons, resulting in a higher concentration of protons in the intermembrane space compared to the matrix.
Generation of ATP; The electrochemical gradient created by the proton pumping is utilized by ATP synthase, an enzyme complex embedded in the inner mitochondrial membrane.
Coupling Electron Transport with Proton Pumping; The pumping of protons across the inner mitochondrial membrane is coupled with the flow of electrons through the electron transport chain.
To know more about mitochondrial membrane here
https://brainly.com/question/31808640
#SPJ4
Why Do Some Things Stop While Others Keep Going?
Answers
Answer:because they run out of energy when it is transferred and to other objects, or their kinetic energy
Explanation:
Which of the following aqueous solution has the highest freezing point?
A. 0.1 M Sucrose
B. 0.01 M NaCl
C. 0.1 M NaCl
D. 0.01 M Na2SO4
Answers
0.01 M NaCl solution will have the highest freezing point
Freezing point will be given by the formula:
ΔT(f) = iK(f).m
where, m = molality
Sucrose is non-electrolyte and so i = 1
NaCl → Na+ + Cl−
Thus i = 2
Na2SO4 → 2Na+ + SO4^2−
Thus i = 3
The highest freezing point will be of the solution having the lowest ΔT(f) value.
As option B has least value of both i and m, 0.01M NaCl has lowest
ΔT(f) = K(f) × (0.02)
Therefore, 0.01 M NaCl solution will have the highest freezing point.
Learn more about Freezing point here:
https://brainly.com/question/40140
#SPJ4
Answer:
0.01 M NaCl is the answer
WORTH 25 POINTS
PLS..!!!!!
Primary consumers _____.
a. receive energy directly from the sun
b. make their own food using photosynthesis
c. break down dead matter into chemical nutrients
d. are the first to receive energy from a plant
Answers
Answer: D. Are the first to receive energy from a plant.
Explanation: Paying attention to the trophic level in which the food chain occurs is key. Producers are the ones that receive energy directly from the sun. They also make their own food using photosynthesis. A decomposer is the one who breaks down dead matter into chemical nutrients. Therefore, Primary consumers are the first to receive energy from a plant.
I hope this helped!
Good luck <3
A metal with a specific heat of 0.780 J/g℃ requires 45.0 J of heat to raise the temperature by two degrees Celsius. What is the mass of the metal?
Answers
Q = mc∆T
45.0 J = (x)(0.780 J/g*C)(2)
x = 28.85 grams
The mass of the metal can be determined using the calorimetric equation. The specific heat of the metal is 0.780 J/degree Celsius and heat required is 45 J. Then, mass of the metal sample is 28.8 g.
What is calorimetry ?Calorimetry is analytical tool used to measure the heat energy absorbed or released by a system. The calorimetric equation of heat energy q with mass m, specific heat c and temperature difference ΔT is:
q = m c ΔT
Given ,
q = 45 J
c = 0.780 J/g℃
then, the mass m in grams is calculated as follows:
m = q/c ΔT
m = 45 J/ 0.780 J/g℃ × 2°C = 28.8 g.
Therefore, the mass of the metal with a specific heat of 0.780 J/g℃ requires 45.0 J of heat to raise the temperature by two degrees Celsius is 28.8 g.
Find more on calorimetry:
https://brainly.com/question/11477213
#SPJ3
What is the molar mass of carbon?
Answers
A sample of hydrogen is collected over water in a eudiometer. The volume is 35.5 mL at a temperature of 21°C and a barometric pressure of 735 mmg. what would the volume of dry hydrogen gas be for the gas at STP?
Answers
Explanation:
To find the volume of dry hydrogen gas at STP, we need to use the combined gas law, which relates the pressure, volume, and temperature of a gas.
The combined gas law is given by:
(P1 V1) / (T1) = (P2 V2) / (T2)
where P1, V1, and T1 are the initial pressure, volume, and temperature of the gas, and P2, V2, and T2 are the final pressure, volume, and temperature of the gas.
First, we need to adjust the initial volume of hydrogen to account for the presence of water vapor. The total pressure in the eudiometer is the sum of the pressure of the hydrogen gas and the pressure of the water vapor, which is equal to the partial pressure of water vapor in the air. The partial pressure of water vapor can be found using a vapor pressure table at the given temperature of 21°C, which is 18.7 mmHg.
Thus, the initial pressure of the hydrogen gas is equal to the total pressure minus the partial pressure of water vapor:
P1 = 735 mmHg - 18.7 mmHg = 716.3 mmHg
Now we can plug in the values into the combined gas law and solve for V2, the volume of the dry hydrogen gas at STP:
(P1 V1) / T1 = (P2 V2) / T2
where P2 = 1 atm (at STP) and T2 = 0°C = 273 K
V2 = (P1 V1 T2) / (T1 P2)
V2 = (716.3 mmHg x 35.5 mL x 273 K) / (294 K x 760 mmHg)
V2 = 29.5 mL
Therefore, the volume of dry hydrogen gas at STP is 29.5 mL.
charged particles, like na⁺ and cl⁻, flow down ____________ gradients when ion channels are open.
Answers
Charged particles, like Na⁺ and Cl⁻, flow down electrochemical gradients when ion channels are open.
When ion channels are open, charged particles such as Na⁺ (sodium ions) and Cl⁻ (chloride ions) move down their electrochemical gradients. An electrochemical gradient consists of two components: an electrical gradient (created by a difference in charge) and a chemical gradient (created by a difference in ion concentration).
These ion channels act as selective pores in the cell membrane, allowing specific ions to pass through. When the channels open, ions move from an area of higher concentration to an area of lower concentration. For example, Na⁺ ions will flow from an extracellular region with a higher concentration of Na⁺ to an intracellular region with a lower concentration of Na⁺. Similarly, Cl⁻ ions will flow from an extracellular region with a higher concentration of Cl⁻ to an intracellular region with a lower concentration of Cl⁻.
This movement down the electrochemical gradients is driven by the principle of diffusion, where particles tend to move from areas of higher concentration to areas of lower concentration until equilibrium is reached.
learn more about electrochemical gradients here:
https://brainly.com/question/13182313
#SPJ4
A 1.03×10−6mol sample of Sr(OH)2 is dissolved in water to make up 25.0 mL of solution. What is the pH of the solution? Round the answer to three significant figures. Select the correct answer below: 4.08, 9.92, 9.61, 8.31
Answers
The pH of the solution is 9.92, The pH of a solution involves calculating the concentration of hydrogen ions in the solution
Sr(OH)2 (s) → Sr2+ (aq) + 2 OH- (aq)The next step is to calculate the concentration of hydroxide ions in the solution using the stoichiometry of the balanced equation. We are given that 1.03×10−6 moles of Sr(OH)2 are dissolved in 25.0 mL of solution, so we can use the following equation to calculate the concentration of hydroxide ions:[OH-] = 2 × (1.03×10−6 mol)/(0.0250 L) = 8.24×10−5 M
Finally, we can use the relationship between the concentration of hydroxide ions and the concentration of hydrogen ions in water to calculate the pH of the solution:pOH = -log[OH-] = -log(8.24×10−5) = 4.08pH = 14 - pOH = 14 - 4.08 = 9.92
To know more about pH visit:-
https://brainly.com/question/2288405
#SPJ11
The useful energy a light bulb gives out is
(a). kinetic energy (b) chemical energy (c) light energy
science subject
Answers
Answer:
Light bulb gives light energy which is usefull
Answer:
(c) light energy
Explanation:
As bulb is used for giving light and hence the energy that it gives is light energy.