Write a balanced half-reaction for the product that forms at each electrode in the aqueous electrolysis of the following salts: (a) ZnBr₂;
Answers
A balanced half reaction for the product that forms at anode is 2H₂O → O₂ + 4H⁺ + 4e⁻ and product that forms at cathode is 2H₂O + 2e⁻ → H₂ + 2OH⁻
What is Balanced Chemical Equation ?The balanced chemical equation is the equation in which the number of atoms on the reactant side is equal to the number of atoms on the product side in an equation.
Now write the half equation
Zn⁺² + 2e⁻ → Zn ....(1)
2H₂O + 2e⁻ → H₂ + 2OH⁻ ...(2)
From equation (1) and (2), we can say that reduction of H₂O will occur at cathode.
2Br⁻ → Br₂ + 2e⁻ ....(3)
2H₂O → O₂ + 4H⁺ + 4e⁻ ...(4)
From equation (3) and (4), we can say that oxidation of H₂O will occur at anode.
At cathode: 2H₂O + 2e⁻ → H₂ + 2OH⁻
At anode: 2H₂O → O₂ + 4H⁺ + 4e⁻
Thus from the above conclusion we can say that A balanced half reaction for the product that forms at anode is 2H₂O → O₂ + 4H⁺ + 4e⁻ and product that forms at cathode is 2H₂O + 2e⁻ → H₂ + 2OH⁻
Learn more about the Balanced chemical equation here: brainly.com/question/26694427
#SPJ4
Related Questions
What makes modern chemistry different from alchemy and other ancient chemical practices of pre-modern humans?
Answers
Answer:
Alchemy was based more on experimentation and had little basis in science. Chemistry utilizes both experimentation and scientific practices. Modern chemistry basically relies on scientific theories and experimental results, but the alchemy was a blend of myths, religion, magic, astrology, philosophy, and spirituality.
If substances B and C are both in the gas phase and are at the same energy level, which of the two substances will need to have more energy transferred out in order to change to the liquid phase? Substance B or Substance C? Explain your answer below.
For Science
Answers
Answer:
Substances can change phase—often because of a temperature change. At low temperatures, most substances are solid; as the temperature increases, they become liquid; at higher temperatures still, they become gaseous.
The process of a solid becoming a liquid is called melting. (an older term that you may see sometimes is fusion). The opposite process, a liquid becoming a solid, is called solidification. For any pure substance, the temperature at which melting occurs—known as the melting point—is a characteristic of that substance. It requires energy for a solid to melt into a liquid. Every pure substance has a certain amount of energy it needs to change from a solid to a liquid. This amount is called the enthalpy of fusion (or heat of fusion) of the substance, represented as ΔHfus. Some ΔHfus values are listed in Table 10.2 “Enthalpies of Fusion for Various Substances”; it is assumed that these values are for the melting point of the substance. Note that the unit of ΔHfus is kilojoules per mole, so we need to know the quantity of material to know how much energy is involved. The ΔHfus is always tabulated as a positive number. However, it can be used for both the melting and the solidification processes as long as you keep in mind that melting is always endothermic (so ΔH will be positive), while solidification is always exothermic (so ΔH will be negative).
Which of the following solids is commonly used as an inactive electrode in electrochemical cells?
a. Zinc.
b. Graphite.
c. Copper.
d. Iron.
e. Sodium.
Answers
The solid commonly used as an inactive electrode in electrochemical cells is Graphite. So option b is the correct answer.
Graphite is often chosen as an electrode material because it is chemically inert, highly conductive, and has a stable structure. This makes it suitable for various electrochemical applications without affecting the overall cell reactions.
An inactive electrode, also known as an inert electrode, is an electrode that does not participate in the chemical reaction occurring in the cell but serves as a conductor for the flow of electrons.
The graphite working electrode, which can be employed as an anode or a cathode in various electrochemical applications, is renowned for its chemical stability, superior electrical conductivity, and high melting point.
So the correct answer is option b. Graphite.
To know more about electrodes: https://brainly.com/question/25749323
#SPJ11
how much does it cost to fill a 100lb propane tank
Answers
Answer:
$500
Explanation:
G00gle.
Question 23
Marks: 1
The rate at which atoms of radioactive sources (radionuclides) disintegrate are measured in
Choose one answer.
a. rems
b. rods
c. curies
d. roentgens
Answers
The rate at which atoms of radioactive sources, or radionuclides, disintegrate is measured in curies. A curie is a unit of measure for the amount of radioactive material present. It represents the amount of radioactive material in which 37 billion atoms disintegrate per second.
The disintegration of radionuclides produces ionizing radiation, which can be measured in rems or roentgens.
A rem is a unit of measurement for the amount of ionizing radiation absorbed by living tissue, while a roentgen is a unit of measurement for the amount of ionizing radiation in the air.
In summary, the rate at which atoms of radioactive sources disintegrate is measured in curies, while the amount of ionizing radiation produced by the disintegration can be measured in rems or roentgens. It is important to understand these units of measurement in order to properly monitor and regulate exposure to ionizing radiation, as it can have harmful effects on living organisms.
The rate at which atoms of radioactive sources (radionuclides) disintegrate is measured in curies (c).
To explain further, radioactive sources contain unstable atoms, called radionuclides. These radionuclides undergo disintegration or decay, during which they emit radiation. To quantify this process, we use various units.
Curies (Ci) is a unit of measurement specifically used to express the activity of a radioactive substance, or how quickly atoms in the radioactive source are disintegrating. One curie represents 37 billion disintegrations per second.
It's important to note the other units you mentioned:
- Rems (roentgen equivalent in man) is a unit used to measure the biological impact of ionizing radiation on human tissue.
- Roentgens (R) is a unit used to measure the exposure to ionizing radiation, specifically the amount of radiation that produces a certain amount of ionization in air.
- Rods is not a unit related to radioactivity, but might be confused with control rods, which are used in nuclear reactors to control the rate of nuclear reactions.
In summary, the appropriate unit for measuring the rate at which atoms of radioactive sources disintegrate is curies.
Learn more about radioactive at : brainly.com/question/1770619
#SPJ11
Step 7: Put the Metal in the Water and Measure
Temperature Changes (Aluminum)
The temperature at 300 seconds (5 minutes) is the
final temperature of both the metal and the water.
Record it to the nearest 0.1°C in the data table.
27
26
25
24
23
05:00
20
Continue (60s more)
www.w
250 mL
223
200
150
100
50
(1-0)
Initial temperature of metal = 100
°C
Initial temperature of water = 22.4
°C
Final temperature of both = 27.1
°C
COMPLETE
Subtract to find the temperature changes
for the water and the metal.
AT (water) =
1°C
AT (metal) =
DONE✔
==
°C
Answers
Change in temperature of the metal, ΔT₁ = 82.9°C
Change in temperature of water, ΔT₂ = 4.7°C
What is temperature?Temperature is a measure of the average kinetic energy of the molecules in a substance.
It is defined as the degree of hotness or coldness of a body.
Temperature changes occur when heat is added or removed from a substance.
To determine the temperature change in water and the meatal when the heated aluminum was placed in water, the following calculations are done:
Initial temperature of metal = 100°C
Initial temperature of water = 22.4°C
Final temperature of both = 27.1°C
Change in temperature of the metal, ΔT₁ = 100 - 27.1
ΔT₁ = 82.9°C
Change in temperature of water, ΔT₂ = 27.1 - 22.4°C
ΔT₂ = 4.7°C
In conclusion, the change in temperature is determined by finding the difference in the temperatures.
Learn more about temperature change at: https://brainly.com/question/24719561
#PSJ1
Answer:
100
22.4
27.1
4.7
72.9
Explanation:
It just is, trust me
Which symbol represents a salt?
O CaCl2
O C6H12O6
O C2H2
O 02

Answers
A) CaCl2
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a colorless crystalline solid at room temperature, highly soluble in water.
How much energy is required to remove a proton from 157n ? the masses of the atoms 157n , 146c and 11h are 15. 000109 u , 14. 003242 u and 1. 007825 u , respectively.
Answers
The energy is required to remove a proton from ¹⁵N₇ is
The reaction to remove the proton is given as follows :
¹⁵N₇ ----> ¹⁴C₆ + H
The mass of ¹⁵N₇ = 15.000109
the mass of ¹⁴C₆ = 14.003242
The mass of H = 1.007825
The energy requires to remove a proton is given as:
E = ( 14.003242 + 1.007825 - 15.000109) u
E = 0.010958 × 9312.5 MeV
E = 10.2046375 MeV
Thus, energy is required to remove a proton from ¹⁵N₇ with the masses of the atoms ¹⁵N₇ , ¹⁴C₆ and H are 15.000109 u , 14.003242 u and 1.007825 u , respectively is 10.2046375 MeV.
To learn more about proton here
https://brainly.com/question/14162227
#SPJ4
In which layer of the ocean do surface currents flow?
Answers
Answer:
ooh sorry, but will this help you now:
Ocean dynamics define and describe the motion of water within the oceans. Ocean temperature and motion fields can be separated into three distinct layers: mixed (surface) layer, upper ocean (above the thermocline), and deep ocean. Ocean currents are measured in sverdrup (sv), where 1 sv is equivalent to a volume flow rate of 1,000,000 m (35,000,000 cu ft) per second.
Surface currents, which make up only 8% of all water in the ocean, are generally restricted to the upper 4…
Explanation:
Hope this helps :)
I NEED HELP!!
When a precipitate is being filtered from a solution, a funnel and a filter paper are used where the solution passes through but the ppt remains.
My question: IS IT POSSIBLE FOR THE PPT TO PASS THROUGH AS WELL FOR ANY REASON? IF SO, WHY?
Answers
1. The filter paper is damaged: If the filter paper is torn or has small holes, some of the precipitate may pass through along with the solution. This can happen if the filter paper is mishandled or if it is not the correct size for the funnel.
2. The filter paper is clogged: If the filter paper becomes clogged with precipitate, it may no longer be able to effectively separate the solution from the solid. This can happen if too much precipitate is added to the filter paper at once, or if the precipitate is particularly fine.
3. The precipitate is too small: If the precipitate particles are very small, they may be able to pass through the pores in the filter paper along with the solution. In this case, a different type of filter paper or a different filtration method may be needed to effectively separate the precipitate.
Overall, while it is possible for precipitate to pass through a filter paper, this is typically a sign that something has gone wrong in the filtration process. It is important to ensure that the filter paper is undamaged, not clogged, and appropriate for the size and type of precipitate being filtered.
*Absolute zero is the temperature when:
Answers
Answer:
It is the temperature at which water is frozen or is pure ice
"Absolutely zero" temperature is the coldest temperature possible. It's so cold that everything stops moving and has no energy left. Scientists use a special scale called Kelvin to measure temperature, and absolute zero is at 0 Kelvin or -273.15 degrees Celsius (-459.67 degrees Fahrenheit). We can't actually reach absolute zero in real life, but scientists have come very close in laboratories using special cooling methods.
if i were to draw an electron configuration for a arsenic (as) atom, what is the highest energy level i would reach?
Answers
4 is the highest energy level in arsenic as the 4s and 4p subshells are found in the fourth energy level. The 4s subshell has two electrons, while the 4p subshell has three. Arsenic thus has a valence electron count of 2 + 3 = 5.
A p-block element with an atomic number of 33 is arsenic.
Arsenic is a member of group 15.
Group 15's electronic configuration:
These elements' valence shell electrical configuration is ns2np3.
These elements' electronic arrangement is exceptionally stable due to their fully filled s orbitals and partially filled p orbitals.
Arsenic's electronic configuration:
3s2 3p6 4s2 3d10 4p3 s2 2s2 2p6
In the above electron configuration, the highest energy level (4).
to know more about electronic configuration visit
https://brainly.com/question/29157546
#SPJ4
PLZ HELP!
Where have you seen or known of examples of waves in your life? Based on science terms of a wave.
Answers
Answer:
I answered this in your recent post of this question.
Explanation:
Hope that this helps you out! :)
If you have any questions please put them in the comment section below this answer.
Have a great rest of your day/night!
Please thank me on my profile if this answer has helped you.
Potassium metal reacts with chlorine gas to form solid potassium chloride. Answer the following:
Write a balanced chemical equation (include states of matter)
Classify the type of reaction as combination, decomposition, single replacement, double replacement, or combustion
If you initially started with 78 g of potassium and 71 grams of chlorine then determine the mass of potassium chloride produced.
Answers
The reaction between pottasium metal and chlorine gas is an example of combination reaction and the balanced equation is as follows: 2K + Cl₂ → 2KCl
What is a chemical equation?A chemical equation is a symbolic representation of a chemical reaction where reactants are represented on the left, and products on the right.
A chemical equation is said to be balanced when the number of atoms of each element on both sides of the equation are the same.
According to this question, potassium metal reacts with chlorine gas to form solid potassium chloride. The balanced equation is as follows:
2K + Cl₂ → 2KCl
Based on the above equation, pottasium combines with chlorine chemically to form pottasium chloride compound, hence, it is an example of combination reaction.
Learn more about combination reaction at: https://brainly.com/question/32027270
#SPJ1
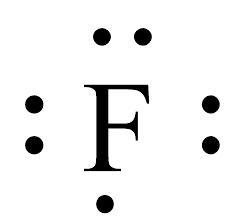
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
why are so many products made from plastic ?
Answers
Explanation:
Plastic takes time to degrade which means it has great longevity. Plastic does not break as easily as glass or other materials. It lasts long and offers great service. Plastic storage containers offer greater flexibility than any other packaging materials.
In a simple distillation setup, what is the sequence of equipment from the bench top to the round bottom flask
Answers
In a simple distillation setup, the sequence of equipment from the bench top to the round bottom flask is:
ThermometerDistillation flaskLiebig condenserRound bottom flaskBunsen burnerWhat is Distillation?This is the process in which a mixture is separated through selective boiling and condensation.
The distillation flask and liebig condenser are usually located above the round bottom flask in the set up.
Read more about Distillation here https://brainly.com/question/24553469
#SPJ4
The point at which indicator undergoes colour change is called end point titration. True or False?
Answers
The given statement "The point at which indicator undergoes color change is called end point titration. " is True.
The endpoint of a titration is the point at which the indicator being used undergoes a color change, indicating that the reaction between the analyte and the titrant is complete. The choice of indicator depends on the nature of the reaction being studied and the pH range in which the reaction occurs. The indicator is selected such that its color changes at the pH at which the reaction is complete. For example, phenolphthalein is commonly used as an indicator in acid-base titrations because it changes from colorless to pink in the presence of a base, indicating the endpoint of the titration.
To know more about phenolphthalein, here
brainly.com/question/14804470
#SPJ4
what is the molar concentration of a solution that contains 45.0 g of nacl dissolved in 350.0 ml of water? question 36 options: 0.00220 m 2.20 m 12.9 m 129 m
Answers
, the molar concentration of the solution is 2.202 M. molar concentration of a solution that contains 45.0 g of nacl dissolved in 350.0 ml of water
To calculate the molar concentration of a solution, we need to first determine the number of moles of the solute present in the solution, and then divide that by the volume of the solution in liters.
The molar mass of NaCl is 58.44 g/mol. Therefore, the number of moles of NaCl in 45.0 g can be calculated as:
mole= mass / molar mass = 45.0 g / 58.44 g/mol = 0.7709 mol
Next, we need to convert the volume of the solution from milliliters to liters:
volume = 350.0 ml = 0.3500
Finally, we can calculate the molar concentration (M) of the solution as:
M = moles / volume = 0.7709 mol / 0.3500 L = 2.202 M
Learn more about concentration here:
https://brainly.com/question/30665317
#SPJ11
What makes plants the beginning of the food chain?
please answer my question aswell as my last one!

Answers
Answer: A
Explanation:
i just took the quiz and it was right!
HELP!!!
What is the balanced chemical reaction when zinc is added to copper sulfate
Answers
the answer should be
Zn+CuSO4→ZnSO4+Cu.
I hope this is right!
What is the % by mass of oxygen in Mg(NO3)2 ?
Answers
Answer:
72.71 %
Explanation:
For oxygen: mass % O = (mass of 1 mol of oxygen/mass of 1 mol of CO2) x 100. mass % O = (32.00 g / 44.01 g) x 100. mass % O = 72.71 %
HOPED THIS HELPED YOU OUT
A brainliest is always appreciated.
What explanation from the evidence within the chemical equation BEST supports the idea that matter is conserved in a chemical reaction? A There are the same number of atoms of oxygen and hydrogen in the reactants as there are in the products. B There are the same number of elements in the reactants as there are in the products. C More reactants, hydrogen and oxygen, were added to make the product, water. D Two reactants, hydrogen and oxygen, combined to make one product, water.
Answers
Answer:
A There are the same number of atoms of oxygen and hydrogen in the reactants as there are in the products.
Explanation:
Matter is conserved in a chemical equation if there are no loss of atoms. That means, total number of atoms of elements must be the same as the total number of element on the product side. The correct option is;
A. There are the same number of atoms of oxygen and hydrogen in the reactants as there are in the products.
Answer:
its A. i just did dat
A sample of CO2 is placed in a sealed rigid cylinder with a movable piton at 81 C and 101KPa. Suggest a change in temperature and change in pressure of the CO2 that would cause it to behave more like an ideal gas
Answers
In order for CO2 to behave more like an ideal gas, a change in temperature and pressure needs to be suggested. If the pressure of the CO2 sample is reduced and the temperature is increased, then the behavior of the gas will be more like an ideal gas.
A sample of CO2 is placed in a sealed rigid cylinder with a movable piston at 81°C and 101 kPa. What is the change in temperature and pressure of the CO2 that would cause it to behave more like an ideal gas An ideal gas follows the laws of Boyle and Charles, where the pressure, volume, and temperature are related by the equation PV=nRT. The ideal gas law assumes that the gas molecules have no volume and do not interact with each other. In reality, no gas behaves exactly like an ideal gas. However, if the pressure is reduced and the temperature is increased, the behavior of the gas will be more like an ideal gas. At a constant volume, the pressure and temperature of a gas are directly proportional.
Therefore, if the pressure is reduced, the temperature must be increased to keep the behavior of the gas constant. The amount of gas in the cylinder is constant, so n does not change. Therefore, the change in pressure and temperature is proportional to the change in volume. The ideal gas law is a fundamental equation that relates the pressure, volume, and temperature of an ideal gas. The law is expressed as PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature. The ideal gas law assumes that the gas molecules have no volume and do not interact with each other.
To know more about gas Visit;
https://brainly.com/question/13123721
#SPJ11
Where in nature would we find pure
sand that does not contain smaller or
larger particles?
Answers
Explanation:
Sand is a common material found on beaches, deserts, stream banks, and other landscapes worldwide. In the mind of most people, sand is a white or tan, fine-grained, granular material. However, sand is much more diverse - even beyond the pink sand beaches of Bermuda or the black sand beaches of Hawaii.
¿por qué la teoría de Demócrito fue reemplazada por la teoría de Dalton y luego esta teoría fue reemplazada por por Rutherford.?
Answers
Answer:
Debido al descubrimiento de protones, neutrones y electrones.
Explicación:
La teoría de Demócrito fue reemplazada por la teoría de Dalton y luego la teoría de Dalton fue reemplazada por la de Rutherford debido al descubrimiento de protones, neutrones y electrones que están presentes dentro del átomo. Demócrito afirma que el átomo es una partícula indivisible de materia, pero el descubrimiento del protón, el neutrón y el electrón cambió este concepto de átomo, por lo que debido al mayor descubrimiento y estudio del átomo y sus partículas subatómicas, las teorías cambiaron con el paso del tiempo.
GIVING BRAINLIEST
What do the arrows at point 3 indicate?
O Cool air is above water.
O Warm air is above land.
O Warm air is cooling quickly.
O Cool air is warming quickly.
Answers
Answer:
D) Cool air is warming quickly.
Explanation:
d is the answer.
The arrows at point 3 indicates that Warm air is cooling quickly and the correct option is option C.
What is Sea Breeze?Sea breeze is a blowing breeze from sea towards land during the day is called sea breeze.
During the day, the land surface heats up faster than the water surface.
Therefore, the air above the land is warmer than the air above the ocean.
As a result, warm air rises. Therefore, the warmer air over the land surface is rising.
As the warm air over the land is rising, the cooler air over the ocean is flowing over the land surface to replace the rising warm air
Therefore, The arrows at point 3 indicates that Warm air is cooling quickly and the correct option is option C.
Learn more about Sea Breeze, here:
https://brainly.com/question/13015619
#SPJ7
When many water molecules form hydrogen bonds with other water molecules, they form a lattice of water molecules, which is strong and flexible. The cohesive forces between liquid molecules are responsible for the phenomenon known as ________________.
Answers
Answer:
KEY TAKEAWAYS
Key Points
The difference in electronegativities between oxygen and hydrogen atoms creates partial negative and positive charges, respectively, on the atoms.
Water molecules attract or are attracted to other polar molecules.
Molecules that do not dissolve in water are known as hydrophobic (water fearing) molecules.
Key Terms
hydrophilic: having an affinity for water; able to absorb, or be wetted by water
hydrophobic: lacking an affinity for water; unable to absorb, or be wetted by water
polarity: The intermolecular forces between the slightly positively-charged end of one molecule to the negative end of another or the same molecule.
Explanation:
maybe this will help :)
gosh i rly need help in the previous questions I asked and no one answered I wish I was smart to answer them alone why am I d.umb
Answers
Answer:
gonna check them rn :) youre not d u mb dont worry (:
Which is the molar mass of BF3?
10.81 g/mol
29.81 g/mol
48.81 g/mol
67.81 g/mol
Answers
Answer: The molar mass of \(BF_3\) is 67.81 g/mol
Explanation:
Molar mass is defined as the mass in grams of 1 mole of a substance.
S.I Unit of Molar mass is gram per mole and it is represented as g/mol.
Atomic Mass of Boron (B) = 10.81 g
Atomic Mass of Flourine (F) = 18.99 g
Molecular mass of \(BF_3\) = 1 (10.81)+3(18.99) g = 67.81 g
Thus molar mass of \(BF_3\) is 67.81 g/mol