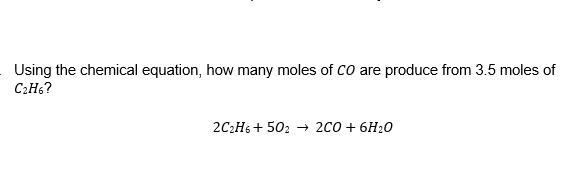Answers
Answer:
3.5 moles CO
Explanation:
To convert between moles of CO and moles of C₂H₆, you need to use the mole-to-mole ratio. These values are the coefficients in the reaction. Because you are starting with C₂H₆, the coefficient of C₂H₆ should be on the bottom of the ratio to allow for the cancellation of units.
2 C₂H₆ + 5 O₂ ----> 2 CO + 6 H₂O
3.5 moles C₂H₆ 2 moles CO
----------------------- x ------------------------ = 3.5 moles CO
2 moles C₂H₆
Related Questions
The total number of atoms represented by the formula (NH4)2Cr2O7 is
a. 23
b. 11
c. 19
d. 18
will give branliest for quick and correct answers
Answers
Answer:
its 19 I'm pretty sure about
Answer:
c is correct and my girl friend
The dipole moment of water is 1. 85D but CO2 has zero dipole moment. Why ?
Answers
Answer:
The dipole moment of a molecule depends on the magnitude and direction of the bond dipoles in the molecule. In water, the two O-H bonds are polar, meaning that they have a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. This creates a net dipole moment for the molecule, with the negative end of the dipole moment located near the oxygen atom and the positive end located near the hydrogen atoms.
In contrast, CO2 has a linear geometry, with two polar C-O bonds oriented in opposite directions. The bond dipoles are equal in magnitude but opposite in direction, so they cancel each other out, resulting in a net dipole moment of zero.
Therefore, the dipole moment of CO2 is zero because the bond dipoles are symmetrically arranged and cancel out each other, while the dipole moment of water is nonzero because the bond dipoles are not symmetrically arranged and do not cancel out each other.
7. Look at the graph in Figure 14.10 on page 420. What two observations did
Jacques Charles make about the behavior of gases from similar data?
Answers
My car has an internal volume of 12,000 L. If I drive my car into the river and it implodes, what will be the volume of the gas when the pressure goes from 1.0 atm to 1.4 atm?
Answers
The volume of gas when the pressure goes from 1.0 atm to 1.4 atm is 8,571.43 L.
When a car is driven into the river, it will implode due to the change in pressure. We are to calculate the volume of gas when the pressure goes from 1.0 atm to 1.4 atm if the internal volume of the car is 12,000 L.In order to solve the problem, we will use the combined gas law equation. The equation is given as follows;P1V1/T1 = P2V2/T2where P1 is the initial pressure, V1 is the initial volume, T1 is the initial temperature, P2 is the final pressure, V2 is the final volume, and T2 is the final temperature.We will assume that the initial temperature and final temperature are constant, and therefore, we can cancel them from the equation. Thus, the equation becomes;P1V1 = P2V2We can rearrange the equation to solve for V2 as follows;V2 = (P1V1)/P2Substituting the given values, we get;V2 = (1.0 atm * 12,000 L)/1.4 atmV2 = 8,571.43 L.
For more questions on pressure
https://brainly.com/question/24719118
#SPJ8
HELP! NEED ASAP
1. A mixture of 11.23 moles of A, 26.50 moles of B, and 45.83 moles of C is placed in a one-liter container at a certain temperature. The reaction is allowed to reach equilibrium. At equilibrium, the number of moles of B is 29.445. Calculate the equilibrium.
(A)- A(g) + B(g) C(g)
(B)- SHOW ALL YOUR STEPS IN THE CALCULATIONS.
Answers
The equilibrium constant of the reaction from the calculation that has been done is 0.154
What is the equilibrium constant?The concentrations (or partial pressures) of reactants and products in chemical equilibrium for a specific chemical reaction are related by the equilibrium constant (K), a mathematical equation. It quantifies the degree to which an equilibrium has been reached in a reaction.
We have the equation of the reaction as;
A(g) + B(g) ⇔C(g)
Thus;
Keq = 45.83/11.23 * 26.50
Keq = 0.154
Learn more about equilibrium constant:https://brainly.com/question/28559466
#SPJ1
How can you tell if an atom is an isotope?
Answers
Answer:
Look up at the atom on the periodic table of elements and find out what its atomic mass is. Subtract the number of protons from the atomic mass. This is the number of neutrons that the regular version of the atom has. If the number of neutrons in the given atom is different, than it is an isotope.
11. Carbon tetrachloride is a solvent which is used as a refrigerant and also as a cleaning agent.
CH4 + 4Cl₂ ⇒ CCl4 + 4HCI
Use the balanced chemical equation above to calculate how many grams of carbon tetrachloride
(CCl4) can be produced from reacting 709.0 grams of chlorine (Cl₂).
Molar Mass Cl₂ = 70.906 g/mol
Molar Mass CCl4 = 153.823 g/mol
a. 3.845 g
b. 61.53 g
384.5 g
6153 g
c.3845 g
d.6153 g
Answers
Answer:
3846g of Carbon tetrachloride is in the chemical equation.
Explanation:
The Balanced equation is :
CH4 + 4CL2 -> CCL4 + 4HCL
By observing the equation There are 4 moles of chlorine react to produce 1 mole of carbon tetrachloride.so, should use the mole ratio to tell the moles of carbon tetrachloride produced, and convert the moles of CCL to grams.Molar Mass of CL2 is 70.906 g/molMolar Mass of CCL4 is 153.823 g/molThe mass of CL2 is 709.0 gramsConverting grams to moles ;
Moles of CL2 = Mass / Molar mass
Molles of CL2 = 709.0g/70.906g/mol => 10 moles
Moles of CCL4 = Moles of CL2 / 4
Moles of CCL4 = 10 moles/ 4 => 2.5 moles
Converting moles of CCL4 to grams:
Mass of CCL4 = Moles of CCL4 x Molar mass of CCL4
Mass of CCL4 = 2.5 moles x 153.823 g/mol => 384.5575 grams
Therefore 384.6 grams of carbon tetrachloride can be produced from reacting 709.0 grams of chlorine.
To know more about CCL4,
brainly.com/question/31315111
brainly.com/question/13199422
We start with 5.00 moles of an ideal monatomic gas with an initial temperature of 126 ∘C. The gas expands and, in the process, absorbs an amount of heat equal to 1300 J and does an amount of work equal to 2200 J .
What is the final temperature Tfinal of the gas?
Use R = 8.3145 J/(mol⋅K) for the ideal gas constant.
Answers
The final temperature of the gas, after absorbing 1300 J of heat and doing 2200 J of work, is approximately 375.45 K.
To find the final temperature (T_final) of the gas, we can use the first law of thermodynamics, which states that the change in internal energy (ΔU) of a system is equal to the heat added (Q) minus the work done (W) by the system:
ΔU = Q - W
Since the gas is ideal and monatomic, the change in internal energy is related to the temperature change (ΔT) through the equation:
ΔU = nC_vΔT
where n is the number of moles and C_v is the molar heat capacity at constant volume.
Rearranging the equations and substituting the given values:
nC_vΔT = Q - W
(5.00 mol)(3/2R)ΔT = 1300 J - 2200 J
(5.00 mol)(3/2)(8.3145 J/(mol⋅K))ΔT = -900 J
Simplifying:
(37.9725 J/K)ΔT = -900 J
ΔT = -900 J / (37.9725 J/K)
ΔT ≈ -23.70 K
Since the initial temperature is 126 °C, we convert it to Kelvin:
T_initial = 126 °C + 273.15 = 399.15 K
Now we can find the final temperature:
T_final = T_initial + ΔT
T_final = 399.15 K - 23.70 K
T_final ≈ 375.45 K
Therefore, the final temperature of the gas is approximately 375.45 K.
Read more on the First law of thermodynamics here: https://brainly.com/question/26035962
#SPJ11
What is your estimate of the strength for each clay type at 50% water content, with DI water as its pore fluid, and with brine in its pore fluid? - Is there a significant difference? If so, what physical mechanism do you think is causing the change in strength? What is the effect of salt on the shear strength of clays?
Answers
The physical mechanism causing the change in strength when using brine as the pore fluid is the presence of salt ions that weaken the interparticle bonds. Salt can reduce the shear strength of clays by increasing the repulsive forces between clay particles.
The strength of clay types at 50% water content can vary depending on whether DI water or brine is used as the pore fluid. Generally, there is a significant difference in strength between the two.
The presence of salt in brine can have an effect on the shear strength of clays. When salt is dissolved in water, it creates ions that can interact with the clay particles. These interactions can lead to the formation of electrical double layers around the clay particles, which can increase the interparticle repulsion and decrease the shear strength of the clay.
On the other hand, when DI water is used as the pore fluid, there is no presence of salt ions to affect the interparticle interactions. As a result, the clay particles can have stronger bonds and higher shear strength compared to when brine is present.
Learn more about interparticle bonds here :-
https://brainly.com/question/4339576
#SPJ11
What do these two changes have in common?
crushing a mineral into powder
picking up a paper clip with a magnet
Select all that apply.
Both are changes of state.
Both conserve mass.
Submit
Both are only physical changes.
Both are chemical changes.

Answers
The appearance and observable qualities of matter are considered to be its physical attributes. Colour, smell, taste, solubility, etc. An attribute that appears during a chemical reaction is known as a chemical property. A few examples include pH, reactivity, and flammability, etc. The correct option is B.
The chemical makeup or content of matter are not altered after a physical transformation. The internal makeup is unaffected as molecules rearrange themselves during this transformation. The chemical attribute is unaffected by a physical change.
Here both crushing a mineral into powder and picking up a paper clip with a magnet are physical changes.
Thus the correct option is B.
To know more about physical changes, visit;
https://brainly.com/question/28742279
#SPJ1
The photograph shows a strip of land with no trees on the inside cl
river. Which action most directly caused this strip of treeless land to form?
A. Water weathered rock and carried the particles here.
B. Tree roots broke up rock into particles here.
C. Glacial ice lifted up the particles and carried them here.
D. Wind carried particles and deposited them here.
Answers
An action which most directly caused this strip of treeless land to form is that: A. water weathered rock and carried the particles here.
What is an erosion?An erosion can be defined as a geological process that involves the wearing out of rock layers, earthen materials (soil) and the transportation of these solid materials by agents of denudation such as:
WaterWindWhat is weathering?Weathering can be defined as process that involves both the physical and chemical breakdown of rock into smaller pieces or fragments called sediments.
Based on this photograph (see attachment) which shows a strip of land with no trees on the inside curve of a river, we can infer and logically deduce that an action which most directly caused this strip of treeless land to form is because water weathered rock and carried the particles here.
Read more on erosion here: brainly.com/question/15663829
#SPJ1

Answer:
A!!!
Explanation:
the formal charge of an atom reflects the electron count of the particular atom within the molecule, and can be determined using the following formula:
Answers
Formal charge = valence electrons - Number of non bonding electrons - number of shared electrons ÷ 2
A formal charge (F.C.) is the charge assigned to an atom in a molecule in the covalent view of bonding, assuming that electrons in all chemical bonds are shared equally between the atoms, regardless of their relative electronegativity.
The formal charge is generally the difference between an atom's number of valence electrons in its neutral free state and the number allocated to that atom in a Lewis dot structure.
When choosing the optimum Lewis structure or also called as the predominant resonance structure for a molecule, it is important to keep the formal charge on each of the atoms as low as feasible.
The following equation can be used to compute the formal charge of an atom in a molecule:
Formal charge = valence electrons - Number of non bonding electrons - number of shared electrons ÷ 2
To know about formal charge
https://brainly.com/question/14851230
#SPJ4
what is the formula for chlorine monobromide
Answers
Based on how to wrire chemical formulae, the formula for chlorine monobromide is BrCl.
What is chlorine mononbromide?Chlorine monobromide is a covalent compound formed from the covalent combination of chlorine and bromine.
The covalent bond is formed from the sharing of electrons between atoms chlorine and bromine.
Br2 + Cl2 ---> 2 BrCl
Tye formula of chlorinr monobromide is BrCl.
It is also known as bromine monochloride.
Therefore, the formula for chlorine monobromide is BrCl.
Learn more about chemical formula at: https://brainly.com/question/2778716
A student combines 364.6 g of HCl with 80 g of NaOH in 5 L of water. What additional volume of H2O must be added to this mixture to yield a solution with a pH of 1? Note that the molar mass of HCl is 35.46 g/mole, while that of NaOH is 40 g/mole.
Answers
Answer:
75L of additional water to have a pH 1 solution
Explanation:
The reaction of HCl With NaOH is:
HCl + NaOH → H₂O + NaCl
By using molar mass of each reactant you can know how many moles will react, thus:
HCl: 364.6g HCl ₓ (1mol / 36.46g) = 10 moles HCl
NaOH: 80g NaOH ₓ (1mol / 40g) = 2 moles NaOH
That means after the reaction will remain in solution, 10-2 = 8 moles of HCl = 8 moles of H⁺ (In water, HCl dissociates as H⁺ and Cl⁻ ions).
A solution with pH = 1 contains:
pH = -log [H⁺]
1 = -log [H⁺]
0.1M = [H⁺]
As molarity, M is the ratio between moles and liters and you want a solution 0.1M having 8 moles of H⁺ you require:
0.1M = 8 moles H⁺ / 80L
As the student combines the solution with 5L of water, you require
75L of additional water to have a pH 1 solutionwhat will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Explain the effect of pressure on the following chemical equation :
\(CaCO_3 = CaO + CO_2\)
2. Explain the effect of concentration in the following equation :
\(3Fe \: +4H_2O= Fe_3 \: O_4 + 4H_2\)
Answers
Answer:
Explanation:
One
Pressure will favor the side with the least number of mols if it the larger number of moles counts only the gases. (Le Chetalier's Principle). In this case both CaCO3 and CaO are not soluble in water. The only gas is CO2. Since it represents the most number of mols the reactant is favored.
More or Less are very troublesome words in beginning science. Make sure you understand their meaning for this question. The more pressure applied, the more the reactants will be favored and the equation shifts left.
Two
Fe and Fe3O4 are both solids. It depends on the form that water takes. Looking it up, it is shown to be in a gas form. That means if you increase the pressure, nothing will happen. That is not your question, but you should note it.
The answer to your question is you increase the concentration of Fe or H2O, the equilibrium will shift right.
If you add more Fe3O4 or hydrogen it will shift left.
Chemists say that if you alter the equilibrium the equation will shift in the opposite direction to compensate for the action you have taken.
For a certain chemical reaction, the standard gibbs free energy of reaction at is. Calculate the equilibrium constant for this reaction. Round your answer to significant digits.
Answers
The equilibrium constant for the reaction is 3.1 * 10-28 for a chemical reaction of standard Gibbs free energy at 149LJ/mole.
Standard Gibbs free energy is the formation of the compound is the change of Gibbs free energy that involves the formation of 1 mole of a substance in its standard state. The relation of Standard Gibbs free energy is
ΔG° = -RT log K
where,
ΔG°= standard Gibbs free energy. that is 149 KJ/mole
K= equilibrium constant
R= gas constant , that is 8.314 J/K mole
T= temperature at 15°C is 283K
We have to convert KJ in J. so 149 KJ/mole becomes 149000J/mole.
putting all the values in the relation of Gibbs free energy,
149000J /mole = -8.314 J/ K .183K .ln K
K= 3.1 * 10 -28
so the equilibrium constant of the reaction is calculated.
To learn more about Standard Gibbs free energy please visit :
https://brainly.com/question/17310317
#SPJ4
In the question the value of standard Gibbs free energy is not given. question is incomplete. Solving this question by putting a certain value.
which of the following elements has the largest first ionization energy? PLEASE HELP

Answers
Answer:
Boron
Explanation:
Hope it helps you......
Answer:
B. Nitrogen
&
D . Boron
Explanation:
both are the element has the largest first ionization energy
5. describe a difference between observations of testing phosphate ion in whole milk and skim milk. determine if there is a difference in the amount of phosphorous between whole and skim milk. explain.
Answers
We would anticipate finding a higher concentration of calcium in whole milk than in skim milk. Whole milk and skim milk phosphate ion testing observations would not significantly differ from one other. There can be a little, but insignificant, difference in phosphorus content between whole and skim milk and dairy milk.
Testing for calcium ions in whole milk and skim milk yields different results: You would anticipate finding a higher concentration of calcium in whole milk than in skim milk. Higher fat content in whole milk includes fat-soluble vitamins like vitamin D. Calcium from the food is more easily absorbed when vitamin D is present. As a result, vitamin D has been added to whole milk, which helps the body absorb and retain calcium. As a result, whole milk often contains more calcium than skim milk.
Difference between whole milk and skim milk phosphate ion testing observations: Whole milk and skim milk phosphate ion testing observations would not significantly differ from one another. Since phosphate ions are a naturally occurring component of milk and are necessary for a number of biological functions, they can be found in both whole milk and skim milk. When milk is processed to create skim milk, the fat content is removed while the majority of the other ingredients, including phosphorus, are kept. As a result, there wouldn't be much of a difference in the concentration of phosphate ions between whole milk and skim milk.
Difference in phosphorus content between whole and skim milk: There can be a little, but insignificant, difference in phosphorus content between whole and skim milk. The quantity of phosphorus, an important mineral contained in milk, remains largely constant throughout all varieties of milk. Due to the removal of the fat part, skim milk, which has had its fat content reduced, may have a somewhat lower concentration of phosphorus than whole milk. This change, however small, is unlikely to have a substantial effect on the dairy milk's overall phosphorus level.
To know more about dairy milk's:
https://brainly.com/question/30721473
#SPJ4
--The question is incomplete, the complete question is:
"Describe a difference between observations of testing calcium ion in whole milk and skim milk. Determine if there is a difference in the amount of calcium between whole and skim milk. Explain. 5. Describe a difference between observations of testing phosphate ion in whole milk and skim milk. Determine if there is a difference in the amount of phosphorus between whole and skim milk. Explain."--
Which is the best definition of
force?
A. a push or pull
B. a change in motion
C. a motion that does not change
Answers
Answer:
A
Explanation:
........................
Answer:
push or pull i think..okay?
What would increase the amount of your carbon dioxide output?
Answers
Answer:
Consuming electricity: Burning fossil fuels emits CO2, with coal releasing twice as much of the gas as petroleum. Worldwide, fossil fuels generate 85 percent of electricity. The number of coal-burning plants will increase as China and India continue to industrialize.
Find w, x, y and z such that the following chemical reaction is balanced. w Ba3 N₂ + xH₂O →yBa(OH)2 + ZNH3
Answers
The values of balanced chemical reaction is w = 1, x = 6, y = 3, and z = 2
To balance the chemical equation:
1. Balancing nitrogen (N):
There are three nitrogen atoms on the left side (Ba₃N₂), so we need to place a coefficient of 3 in front of NH₃:
w Ba₃N₂ + x H₂O → y Ba(OH)₂ + 3 z NH₃
2. Balancing hydrogen (H):
There are six hydrogen atoms on the left side (2 × 3), so we need to place a coefficient of 6 in front of H₂O:
w Ba₃N₂ + 6 H₂O → y Ba(OH)₂ + 3 z NH₃
3. Balancing barium (Ba):
There are three barium atoms on the left side (3 × Ba₃N₂), so we need to place a coefficient of 3 in front of Ba(OH)₂:
w Ba₃N₂ + 6 H₂O → 3 y Ba(OH)₂ + 3 z NH₃
4. Balancing oxygen (O):
There are six oxygen atoms on the right side (6 × OH), so we need to place a coefficient of 3 in front of Ba(OH)₂:
w Ba₃N₂ + 6 H₂O → 3 Ba(OH)₂ + 3 z NH₃
Now the equation is balanced with the following coefficients:
w Ba₃N₂ + 6 H₂O → 3 Ba(OH)₂ + 3 z NH₃
Therefore, w = 1, x = 6, y = 3, and z = 2 would satisfy the balanced chemical equation.
Learn more about balanced chemical reactions at https://brainly.com/question/26694427
#SPJ11
which particle has the least mass
a. electron
b. proton
c. neutron
d. all have the same mass
Answers
Answer:
b. proton
Explanation:
The mass of an electron is only about 1/2000 the mass of a proton or neutron, so electrons contribute virtually nothing to the total mass of an atom. Electrons have an electric charge of −1, which is equal but opposite to the charge of a proton, which is +1.
Humans have altered the composition
of Earth's atmosphere by
A. Making the air pressure drop
B. Making the air temperature drop
C. Polluting it with excess ozone
D. Polluting it with carbon dioxide gas
Answers
The answer is most likely D
Predict how oxygen saturation would be affected if an individual has defective hexokinase enzymes. a. 2,3-BPG levels are elevated and o b. 1,3-BPG levels are reduced and c. 2,3-BPG levels are elevated and oxy d. 2,3-BPG levels are reduced and oxygen binding ou e 2,3-BPG levels are reduced and oxygen binding increase levels are elevated and oxygen binding decreases to levels are reduced and oxygen binding increases. PG levels are elevated and oxygen binding increases. and oxygen binding decreases
Answers
Oxygen saturation would be affected if an individual has defective hexokinase enzymes is 2,3 - BPG levels are reduced and oxygen binding increases.
Oxygen saturation can be defined as the measure of amount of hemoglobin bound with the oxygen and comparted to the amount of hemoglobin unbounded to the oxygen atom. 2,3 BPG allows the hemoglobin to increase the amount of oxygen. 2,3 bpg is 2.3 Bi phospho glycerate. it increases the supply of oxygen to the tissues by hemoglobin binding.
Thus, Oxygen saturation would be affected if an individual has defective hexokinase enzymes is 2,3 - BPG levels are reduced and oxygen binding increases.
To learn more about oxygen saturation here
https://brainly.com/question/28026966
#SPJ4
Calculate the power developed in R1.
P1 =_____watts
48
190
240
1300
Answers
the answer will be 190
Explanation:
because R1= 0.5*0.5*8
=190
A calculation based upon _______is only as accurate as the device used for measurement.
Answers
The setup in the diagram is left outside during the day and night . Bubbles are continuously produced regardless of the presence of sunlight.what can you predict of the composition in the bubbles
A. The bubbles are always O2
B. The bubbles are always carbon dioxide CO2
C. During the day the bubbles are CO2 and in the night O2
D. During the day they are O2 and in the night CO2
Answers
Answer:
A
Explanation:
The bubbles formed from the plants are always oxygen. The photosynthetic reaction taking place in the plants release oxygen molecules.
What is photosynthesis ?Photosynthesis is the biochemical process of synthesizing chemical energy by green plants with the aid of light energy. They store this chemical energy in the form of glucose.
In photosynthetic reaction, water and carbon dioxide are combined to produce glucose and oxygen gas. The bubbles formed in the reaction is oxygen gas.
This process is an event in the carbon cycle to balance the amount of carbon dioxide in the atmosphere and in living matter. Photosynthesis provide the sufficient oxygen for respiration for animals. Hence, option A is correct.
To find more on photosynthesis, refer here:
https://brainly.com/question/29764662
#SPJ2
A flagpole is an example of what type of pulley system?
A. Movable pulley
B. Fixed pulley
C. Block and tackle?
Answers
Answer:
B
Explanation:
i learned the pulley system in school
Answer:
B. Fixed pulley
Explanation:
Which of the following are possible characteristics for the element this atom represents?
A.
Hard, can be flattened by a hammer
B
A non-reactive colorless gas
C.
Dull, non-conductor of heat and electricity
D
Shiny silver, can be coiled into a wire

Answers
Answer:
B
Explanation:
Sorry if i'm wrong