why is the reaction order for crystal violet the same regardless of concentration of naoh
Answers
The changes in the NaOH concentration will not have an impact on the overall reaction order of crystal violet.
The reaction order for crystal violet is the same regardless of the concentration of NaOH because the reaction follows an overall elementary mechanism that is independent of the NaOH concentration. The reaction order refers to the relationship between the concentration of reactants and the rate of the reaction. In the case of crystal violet, the reaction order is determined by the rate-determining step of the reaction.
The reaction between crystal violet and NaOH typically involves a single-step process, where crystal violet reacts with NaOH to form a product. The rate of this reaction is determined by the concentration of crystal violet, and the concentration of NaOH does not affect the reaction rate. As a result, the reaction order for crystal violet remains the same regardless of the NaOH concentration.
The reaction order being independent of the NaOH concentration suggests that the rate-determining step does not involve any interaction or dependence on the concentration of NaOH. Therefore, changes in the NaOH concentration will not have an impact on the overall reaction order of crystal violet.
Know more about Reaction's Order here:
https://brainly.com/question/1769080
#SPJ11
Related Questions
Chrysanthemic acid occurs as a mixture of esters in flowers of the chrysanthemum (pyrethrum) family. Reduction of chrysanthemic acid to its alcohol, followed by conversion of the alcohol to its tosylate, gives chrysanthemyl tosylate. Solvolysis of the tosylate gives a mixture of artemesia and yomogi alcohols. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism.
Answers
To learn more about chrysanthemic acid visit:
https://brainly.com/question/22985158
#SPJ4

can someone please help me with this question for chemistry. What is the number of moles in 1216 g Sr3(PO4)2? the 3,4, and 2 and small numbers that go under the letters btw
Answers
The primary constituents for the organic molecules needed for life do NOT include which of these compounds
Answers
The primary constituents for the organic molecules needed for life do NOT include inorganic compounds. Organic molecules are composed of carbon atoms and typically contain hydrogen atoms as well. They are found in living organisms and play a crucial role in various biological processes.
Inorganic compounds, on the other hand, do not contain carbon atoms bonded to hydrogen atoms. Examples of inorganic compounds include minerals, metals, salts, and gases like water (H2O) and carbon dioxide (CO2). While inorganic compounds are essential for life, they are not considered primary constituents for the organic molecules needed for life.
The exclusion of inorganic compounds from the list of primary constituents for organic molecules can be illustrated by considering the four major types of organic molecules found in living organisms: carbohydrates, lipids, proteins, and nucleic acids. These molecules are made up of carbon and hydrogen atoms, along with other elements like oxygen, nitrogen.
To know more about inorganic compounds visit :-
https://brainly.com/question/29860925
#SPJ11
Which element will reduce Mg2+ to Mg?
a) Fe b) Ba c) Pb d) Ag
Answers
Answer:
Ba
Explanation:
Let us consider the electrode potential of one of the options listed in the question,
Ba2+(aq) + 2 e- -----> Ba(s). -2.90V
It is very important to recall that a metal can only displace another metal from aqueous solution if that metal has a more negative reduction potential that the metal it displaced. This implies that if there are two metals A and B, A can only displace B from an aqueous solution of B if A has a more negative reduction potential than B.
The reduction potential of Mg^2+ is -2.37V. This is less negative than that of Ba^2+ which is -2.90V. Hence barium can reduce Mg^2+ to metallic Mg.
help please i need it later 11:59pm

Answers
Answer:
In the attached image.
Explanation:
DNA to mRNA codes as:
A-U
U-A
G-C
C-G
(Normally Adenine would code to Thymine but when transferring it to mRNA and above, Adenine codes to Uracil, replacing Thymine).
An Amino Acid is coded from the mRNA.

Calcium + magnesium sulfide
Answers
Answer:
Calcium and magnesium sulphide react to form calcium sulphide and magnesium metal.
Explanation:
Ca + MgS → CaS + Mg
The reaction between calcium and magnesium sulfide produces calcium sulfide and magnesium metal. The balanced equation can be given as Ca + MgS → CaS + Mg.
What is balanced chemical equation?
An equation for just a chemical reaction is said to be balanced if both the reactants as well as the products have the same number of atoms and total charge for each component of the reaction. In other words, both side of the reaction have an equal balance of mass and charge.
Conservation of charge as well as mass, equation and reaction balance, etc. The components and outcomes of a chemical reaction are listed in an imbalanced chemical equation, but the amounts necessary to meet the conservation of mass are not specified. The reaction between calcium and magnesium sulfide produces calcium sulfide and magnesium metal. The balanced equation can be given as Ca + MgS → CaS + Mg.
Therefore, the balanced equation can be given as Ca + MgS → CaS + Mg.
To know more about balanced chemical equation, here:
https://brainly.com/question/15052184
#SPJ2
Decide whether each proposed multiplication or division of measurements is possible. If it is possible, write the result in the last column of the table.

Answers
The possible options for the proposed multiplication or division of measurements are:
1. 9.0 km * 7.0 km = 63 km²; this is a correct option because km² is dimensionally correct.
3. 24 dL²/0.60 L = 0.24 L²/0.60 L = 0.4 L is a correct option since L is a unit of volume.
What are the possible options among the proposed multiplication or division of measurements?The possible options among the proposed multiplication or division of measurements are those which are dimensionally correct.
Considering the given options:
1. 9.0 km * 7.0 km = 63 km²
This is a correct option because km² is dimensionally correct as it is a unit of area.
2. 2.0 g² * 0.043 kg is not correct since there is no unit of square mass.
3. 24 dL²/0.60 L = 0.24 L²/0.60 L = 0.4 L is a correct option since L is a unit of volume.
In conclusion, the correct measurements options are those that are dimensionally correct.
Learn more about measurements at: https://brainly.com/question/777464
#SPJ1
Give a reason why a large number of elk in Yellowstone Park could affect the number of beavers.
Answers
Answer:
Explanation:
A large number of elk in Yellowstone Park could affect the number of beavers due to competition for resources. Here's a detailed explanation:
Habitat Destruction: Elk are herbivores that graze on vegetation, including willow and aspen trees, which are important food sources for beavers. If the elk population is high and they consume a significant amount of vegetation, it can lead to habitat destruction for beavers. With a reduced availability of suitable food and habitat, beaver populations may decline.
Altered Vegetation Dynamics: When elk consume a large amount of vegetation, it can alter the structure and composition of plant communities. This can result in changes in the availability and quality of food sources for beavers. If the preferred vegetation for beavers declines due to heavy grazing by elk, it can negatively impact beaver populations.
Predation Risk: An abundance of elk can attract predators such as wolves and bears, which are known to prey on elk. Increased predation pressure can lead elk to alter their behavior and movement patterns, potentially avoiding areas with higher predator activity. This avoidance behavior by elk can indirectly benefit beavers by reducing the risk of predation. As a result, beaver populations may benefit from the reduced presence of predators associated with high elk populations.
It is important to note that the relationship between elk and beavers is complex and can be influenced by various factors such as ecological interactions, habitat conditions, and predator-prey dynamics. Therefore, the impact of elk on beaver populations in Yellowstone Park may vary depending on specific circumstances and ecosystem dynamics.
Which shows an isomer of the molecule below?

Answers
Answer: C
Explanation:
Don’t trust the other person it’s not A
*view photo for answer*
pay attention! the answer choices aren't always in the same order!

3.0 mol Na reacts with 1.4 mol
F2 according to the equation below:
2Na+ F₂ → 2NaF
How many moles of NaF form
from 3.0 mol Na?
Answers
The number of moles of NaF that will be produced from 3moles of Na is 3 moles.
How to calculate number of moles?Stoichiometry is the study and calculation of quantitative (measurable) relationships of the reactants and products in chemical reactions (chemical equations).
According to this question, sodium reacts with fluorine as follows:
2Na+ F₂ → 2NaF
Based on the above equation, 2 moles of Na will produce 2 moles of NaF
This means that 3 moles of Na will produce 3 moles of NaF.
Learn more about stoichiometry at: https://brainly.com/question/9743981
#SPJ1
Calculate the molality for 22.0g fecl2 in 27.0g h20
Answers
Answer:
6.44 mol/kg
Explanation:
Calculate the molality for 22.0g fecl2 in 27.0g h20
Molality (m) is defined as the number of moles of solute per kilogram of solvent.
To calculate the molality of a solution containing 22.0 g of FeCl2 in 27.0 g of H2O, we first need to convert the given masses of solute and solvent into their respective amounts in moles.
The molar mass of FeCl2 can be calculated as follows:
FeCl2 = 1 x 55.85 + 2 x 35.45 = 126.75 g/mol
The number of moles of FeCl2 is:
nFeCl2 = mass / molar mass = 22.0 g / 126.75 g/mol = 0.1736 mol
The molar mass of H2O is:
H2O = 2 x 1.01 + 16.00 = 18.02 g/mol
The number of moles of H2O is:
nH2O = mass / molar mass = 27.0 g / 18.02 g/mol = 1.499 mol
Now that we have the amounts of solute and solvent in moles, we can calculate the molality using the following formula:
molality = moles of solute / mass of solvent in kg
To express the mass of solvent in kilograms, we divide by 1000:
mass of solvent in kg = 27.0 g / 1000 = 0.027 kg
Now we can calculate the molality:
molality = 0.1736 mol / 0.027 kg = 6.44 mol/kg
Therefore, the molality of the solution containing 22.0 g of FeCl2 in 27.0 g of H2O is 6.44 mol/kg.
Would a rollercoaster have the greatest kinetic energy at the top of the highest hill or at the bottom on the highest hill
Answers
Answer:
The rollercoaster has the lowest kinetic energy at the top of the hill.
The rollercaoster has the highest kinetic energy at the bottom of the hill.
Explanation:
The kinetic energy of any solid body in motion is usually computed using this formula:
K.E = \(\frac{1}{2}mv^2\)
From this, we can see that it varies based on two major parameters - The mass of the moving object and the velocity of the moving object.
We can assume that the mass of the rollercoaster is constant since no one gets off and it does not shrink in its size during the ride.
This means that the variations in the K.E are mainly coming from its velocity.
At the top of the hill, the rollercoaster is moving at its slowest pace. hence, it has the lowest kinetic energy at the top of the hill.
However, at the bottom of the hill, the rollercoaster is moving its fastest, hence it has the highest kinetic energy at the bottom of the hill.
Please help me ASAP
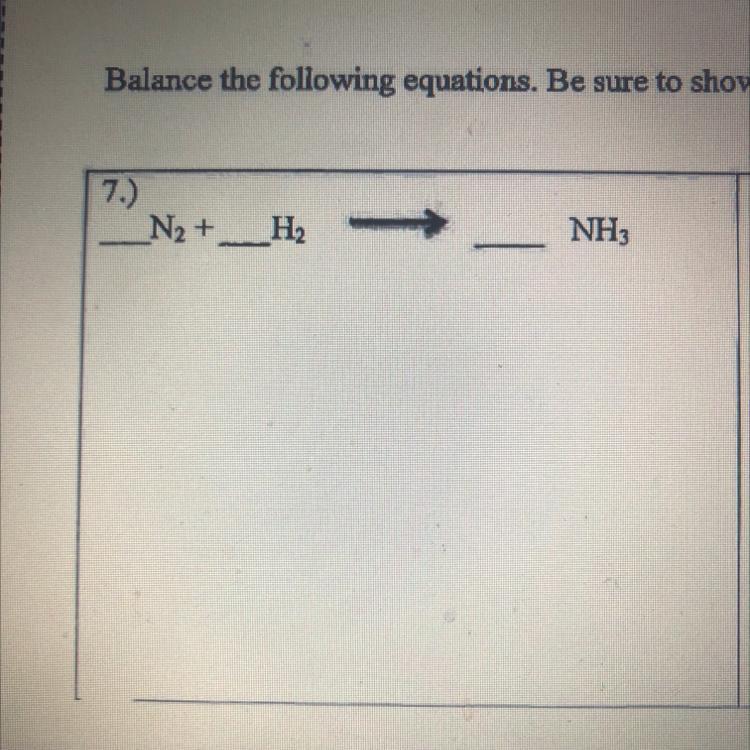
Answers
What type of bond is formed between the hydroxyl group of one nucleotide and the phosphate group of an adjacent nucleotide, forming the sugar-phosphate backbone of dna?.
Answers
Answer:
Phosphodiester bond is the type of bond is formed between the hydroxyl group of one nucleotide and the phosphate group of an adjacent nucleotide.
What is Phosphodiester bond?
This is the type of bond that occurs when two of the hydroxyl groups in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds.
This type of bond is present in the DNA of organisms and forms sugar-phosphate backbone.
Give the number of Particles in:
a. 789 g of Silicon tetrafluoride
b. 0.89g of SI2
Answers
A banana slug's acceleration, which causes it to accelerate by 3.0 feet per minute in 10 seconds. Megabits per second is the current standard for speed, up from kilobits previously. ... x Equals 0 m, where she after 20 seconds of running.
What is acceleration, exactly?An item that follows a circular course while maintaining a constant speed is still moving forward as the source of its motion is shifting. the speed at which velocity varies in respect to time.
Describe the magnitude?It's used to describe something's size or scope.
To know more about acceleration visit :
https://brainly.com/question/12550364
#SPJ4
there are 8 isomeric alcohols with the formula C5H12O. draw the structure of this isomer: 3-methyl-2-butanol.
Answers
The formula C5H12O indicates that there are 5 carbon atoms, 12 hydrogen atoms, and 1 oxygen atom in the molecule.
To draw the structure of 3-methyl-2-butanol, we need to know that the name tells us there is a methyl (CH3) group on the third carbon atom, and that the molecule is a type of alcohol (ending in -ol) with a total of four carbon atoms in a chain, with a hydroxyl (-OH) group attached to the second carbon atom.
To draw the structure, we start by drawing a chain of four carbon atoms, with the second carbon atom having the -OH group attached to it. Then we add a methyl group (CH3) to the third carbon atom. Finally, we add enough hydrogen atoms to satisfy the valences of each atom, keeping in mind that each carbon atom needs four bonds and each hydrogen atom needs one bond. The resulting structure looks like this:
CH3
|
H--C--OH
|
H--C--H
|
H--C--H
|
H
This is the structure for 3-methyl-2-butanol, which is one of the eight isomeric alcohols with the formula C5H12O.
Visit here to learn more about isomeric alcohols : https://brainly.com/question/14718203
#SPJ11
The solubility of copper(II) iodate in water is 0.13 g/ 100 mL. Calculate the solubility product constant for copper(II) iodate, Cu(IO3)2? Cu(IO3)2(s) Cu2+(aq) + 2IO3 - (aq
Answers
The solubility product of the copper(II) iodate is 1.25 * 10^-7
What is solubility?The term solubility has to do with the amount of a substance that is dissolved in water. The solubility product is the equilibrium constant that shows the extent to which a substance is dissolved in water.
We have to convert the solubility of the copper(II) iodate from g/mL to molarity and we have;
Number of moles = 0.13 g/413 g/mol = 3.15 * 10^-4 moles
Volume of the solution = 100 mL or 0.1 L
Molarity = 3.15 * 10^-4 moles/0.1 L
= 3.15 * 10^-3 M
We now have;
Ksp = [x] [2x]^2
Ksp = 4x^3
Ksp = 4( 3.15 * 10^-3 )^3
Ksp = 1.25 * 10^-7
Learn more about solubility:https://brainly.com/question/28170449
#SPJ1
How does temperature affect physical and chemical changes ?
Answers

Explain how the cohesive and adhesive properties of water are useful in maintaining various life processes.
Answers
Answer:
Water molecules' adhesion aids plants in moisture absorption at their roots. Water's initial boiling point is attributed to cohesion, which helps animals regulate their body temperature.
Explanation:
Indique cuáles son las unidades de la molaridad *
eq-g/m³
eq-g/L
mol/cm³
mol/L
Answers
Answer:
hm
Explanation:
predict the two major organic products of the reaction. ( hi behaves as an hx reagent.)
Answers
1. Hydrohalogenation: In this reaction, an alkene reacts with HI (hydroiodic acid), and the hydrogen (H) from HI adds to one carbon of the double bond, while the iodine (I) adds to the other carbon. This results in the formation of an alkyl iodide. The addition follows Markovnikov's rule, which means that the hydrogen will attach to the carbon with more hydrogen atoms, while the iodine will attach to the carbon with fewer hydrogen atoms.
2. Nucleophilic substitution: When HI reacts with an alkyl halide, the iodide ion (I-) from HI acts as a nucleophile and replaces the existing halogen atom on the alkyl halide, resulting in the formation of a new alkyl iodide.
To summarize, the two major organic products of the reaction involving HI as an HX reagent are:
1. Alkyl iodides formed through hydrohalogenation of alkenes
2. Alkyl iodides formed through nucleophilic substitution of alkyl halides.
Learn more about products here : brainly.com/question/31859289
#SPJ11
"What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN) 2 - forms? K sp for AgCl is 1.8 × 10 ^-10 and K f for Ag(CN) 2 - is 1.0 × 10^ 21.
0.050 M
0.10 M
0.40 M
0.20 M"
Answers
The answer is 0.050 M. The molar solubility of a compound depends on its solubility product (Ksp), which is the equilibrium constant for the dissociation of the compound into its constituent ions in water.
What is Molar Solubility?
Molar solubility is defined as the number of moles of a solute that can dissolve in one liter of solvent (usually water) to form a saturated solution at a particular temperature and pressure. It is typically denoted by the symbol "s" and has units of mol/L.
Substituting Ksp and Kf into the expression for x:
Ksp = [Ag+][Cl-] =\(x^{2}\)
Kf = [\(Ag(CN)^{2}\)-]/[Ag+]\([CN-]^{2}\)
[Ag(CN)2-] = [Ag+]
x = [Ag+]
x^2 = Ksp = 1.8 × \(10^{-10\)
x = √(1.8 × \(10^{-10}\)) = 1.34 × \(10^{-5}\)
[Ag(CN)2-] = 5.6 ×\(10^{-7}\) M
Since [Ag(CN)2-] = [Ag+], the molar solubility of AgCl in the presence of 0.10 M NaCN is:
x = [Ag+] = [Ag(CN)2-] = 5.6 × \(10^{-7}\) M
Therefore, the answer is 0.050 M.
Learn more about Molar Solubility from the given link
https://brainly.com/question/28202068
#SPJ4
How many dots would be drawn for the Lewis Dot Structure of gallium?
Answers
Answer:
To draw a Lewis dot structure for an atom, you must know how many valence electrons an atom possesses. The periodic table organizes the elements based on the similarity of their chemical properties.
Explanation:
silver has an atomic mass of 107.868 amu. silver has two common isotopes. one of the isotopes has a mass of 106.906 amu and a relative abundace of 51.881%. a. what is the % abundance of the other isotope. b. what is the mass if the other isotope.
Answers
A. The abundance of the 2nd isotope is 48.119%
B. The mass of the 2nd isotope is 108.905 amu
Let the 1st isotope be A
Let the 2nd isotope be B
A. Determination of the abundance of the 2nd isotope
Abundance of isotope A = 51.881%.
Abundance of isotope B =?Abundance of B = 100 – A
Abundance of B = 100 – 51.881
Abundance of B = 48.119%B. Determination of the mass of the 2nd isotope
Atomic mass of silver = 107.868 amu.
Mass of 1st isotope (A) = 106.906 amu
Abundance of isotope A (A%) = 51.881%.
Abundance of isotope B (B%) = 48.119%
Mass of 2nd isotope (B) =?\(atomic \: mass = \frac{mass \: of \:A \times \:A\%}{100} + \frac{mass \: of \:B \times \:B\%}{100} \\ \\ 107.868 = \frac{106.906\times \ \: 51.881}{100} + \frac{mass \: of \:B \times \:48.119}{100} \\ \\ 107.868 = \: 55.464 + 0.48119 \times mass \: of \:B \\ \\ collect \: like \: terms \\ \\ 0.48119 \times mass \: of \:B = 107.868 - 55.464 \\ \\ divide \: both \: side \: by \: 0.48119 \\ \\ mass \: of \:B = \frac{107.868 - 55.464 }{0.48119} \\ \\ mass \: of \:B =108.905 \: amu \\ \\ \)
Therefore, the mass of the 2nd isotope is 108.905 amu
Learn more: https://brainly.com/question/7955048
N_{2}(g) + 3H_{2} * (g) < =2NH 3 (g)+heat What will happen to equilibrium if the temperature decreases?

Answers
ANSWER
The arrow will be shifted to the right (OPTION B)
EXPLANATION:
Firstly, we need to write out the chemical reaction equation
\(N_{2(g)}+3H_{2(g)}\text{ }\rightleftarrows2NH_{3(g)}\text{ + heat}\)From the reaction above, you will see that heat is one of the products, this means that the reaction is n exothermic reaction.
An exothermic reaction is a type of reaction in which heat is released to its surroundings.
In an exothermic reaction, a decrease in temperature will shift the equilibrium to the right
write electronic configuration of chlorine in its ionic state?
Answers
Answer:
1s2 2s2 2p6 3s2 3p6
Explanation:
Chlorine is a groups 17 element. The halogens for ions by accepting one electron to form univalent negative ions.
Since chlorine normally contains seventeen electrons, the chloride ion consists of eighteen electrons.
Hence the electronic configuration of chlorine ion is; 1s2 2s2 2p6 3s2 3p6.
O2 is paramagnetic and F2 has the highest bond length among the given diatomic compounds.
- C2, N2 and F2 have no unpaired electrons, so they are diamagnetic in nature.
- Only O2 has unpaired electrons in it, so it is paramagnetic in nature.
- Since F atom is very small in size, there is greater electronic repulsion between the F atoms.
- Hence they repel each other and bond length increases.
- So, F2 has the highest bond length among them.
Answers
O2 is paramagnetic and F2 has the highest bond length among the given diatomic compounds.
Since C2, N2, and F2 have no unpaired electrons they are inherently diamagnetic. Only O2 contains unpaired electrons and is therefore paramagnetic. The strongest bond of diatomic species is that of carbon monoxide. The larger the element, the less the lone pair repulsion between the diatomic atoms of the diatomic molecule and the greater the binding energy.
F2 is very low due to the small atomic size of fluorine, which keeps the electrons in a compact volume and strong repulsion between non-bonded electrons. Therefore, it has weaker bonds and the lowest dissociation energy among other halogens. Nitrogen is more stable than oxygen because the bond order of nitrogen is higher than that of oxygen.
Learn more about Diatomic compounds here:-https://brainly.com/question/27299241
#SPJ4
How does the presence of a noncompetitive inhibitor affect the maximum reaction velocity (Vmax) of an enzyme?
Answers
The presence of a noncompetitive inhibitor affects the maximum reaction velocity (Vmax) of an enzyme by decreasing it.
Noncompetitive inhibitors bind to the allosteric site of an enzyme, which is different from the active site where the substrate binds. This binding causes a conformational change in the enzyme, which reduces its activity and decreases the Vmax. Unlike competitive inhibitors, which compete with the substrate for the active site, noncompetitive inhibitors can bind to the enzyme-substrate complex and inhibit the reaction. This means that even if there is a high concentration of substrate available, the Vmax will still be decreased because the inhibitor is binding to the enzyme.
The inhibition caused by a noncompetitive inhibitor is not reversible by increasing the concentration of substrate because the inhibitor is not competing for the same site. The only way to reverse the inhibition is by removing the inhibitor from the system.
Overall, the presence of a noncompetitive inhibitor can significantly decrease the Vmax of an enzyme by altering its conformation and reducing its activity. This can have important implications in biological systems where enzymes play crucial roles in metabolic pathways and other cellular processes.
Know more about Vmax here :
https://brainly.com/question/16108691
#SPJ11
Which of the following can be highly compressed?
a an iron bar
b milk
C liquid water
d nitrogen gas
Answers
Explanation:
d. nitrogen gas because of weak intermolecular force
Vicente is going to spend the afternoon in his yard. He has the choice to get in the pool, lay on a cotton chaise longue, sit on the porcelain tile bordering the pool, or sit on a plastic chair. The specific heats of these items are: Water = 4. 19 J/g•°C Cotton = 1. 30 J/g•°C Porcelain = 1. 07 J/g•°C Plastic = 1. 67 J/g•°C Based on the specific heat values given, where is the coolest location for Vicente to be? pool cotton chaise lounge porcelain tile plastic chair.
Answers
Answer:
A
Explanation: