Why is it important that the di water you use to rinse is ice-cold?.
Answers
It is important that the di water you use to rinse is ice-cold because it helps prevent the formation of water spots. Water spots are formed by impurities that are left behind after the water evaporates.
By using ice-cold water, the impurities don't have time to settle on the surface and create spots before the water evaporates. Additionally, cold water rinses can help to lower the temperature of the substrate being rinsed, which can prevent or slow down the dissolution of particles. This is important in semiconductor manufacturing, where a clean surface is crucial to the performance of the final product.
You can learn more about impurities at: brainly.com/question/32369276
#SPJ11
Related Questions
What volume will 3.50 mol of ammonia gas occupy at conditions of standard temperature pressure?
A 5.41L
B 10.5 L
C 78.4L
D 7,940L
Answers
Answer:
C - 78.4L
Explanation:
Took the test.
What is covalent bond give suitable example?
Answers
Example: Water is made as a result of the covalent bond between hydrogen and oxygen atoms.
Please mark brainliest
what is the formula of Aluminum permanganate
Answers
Answer:
Explanation:
Thus the chemical formula for aluminium permanganate is formed by cross exchanging the coefficient of valencies of aluminium and permanganate (with each other). Thus the chemical formula for aluminium permanganate is represented as : Al(MnO4)3.
A compound that occupies a receptor but does not activate the neuron is known as a(n)?
Answers
A compound that binds to a receptor but does not activate the neuron is known as an Antagonist.
A receptor is a large protein molecule on a neuron that gets activated when a ligand binds to it such as a drug or hormone, or when electrical impulses pass through it.
An antagonist is a drug or hormone that binds to receptor, but instead of activating the receptor, it blocks or dampens the activation of the neuron. Antagonist drugs are used to interfere with the normal function or operation of a protein receptor.
Depending on the nature of the antagonist or the receptor it's bound to, the effects of antagonists may be permanent or temporary.
Learn more about antagonists here:
https://brainly.com/question/11985070
#SPJ4
Which of the following are end-products of glycolysis?
a. CO2 and H2O
b. Pyruvate, CO2, and ATP
c. Pyruvate, NADH, and ATP
d. Acetyl CoA, CO2, and NADH
e. Citrate, H2O, and FADH 2
Answers
The end-products of glycolysis include Pyruvate, NADH, and ATP. Therefore, option C is the correct answer.
Glycolysis is the metabolic pathway that occurs in the cytoplasm of the cell, which plays a vital role in the energy production process. It is also known as the Embden-Meyerhof pathway, and it takes place in the absence of oxygen during cellular respiration.Glycolysis involves the breakdown of glucose into two three-carbon molecules called pyruvate. During the process, a net amount of two molecules of ATP, two molecules of NADH, and two molecules of pyruvate are produced.
The pyruvate is then transported to the mitochondria, where it is further broken down to generate more ATP molecules.
The overall reaction of Glycolysis is:
\(Glucose + 2 NAD^+ + 2 ADP + 2 Pi ⇒2 pyruvate + 2 ATP + 2 NADH + 2 H^+ + 2 H_2O\)
Therefore, The end-products of Glycolysis include: Two molecules of Pyruvate. Two molecules of ATP (Adenosine Triphosphate) are produced. NADH (Nicotinamide Adenine Dinucleotide), which is a high-energy electron carrier, is also produced in glycolysis. hence c option is correct .
To know more about Glycolysis refer here :
https://brainly.com/question/30591040
#SPJ11
1) Carbon atoms are larger than Sodium atoms. (2pt
O True
O False
Answers
True, carbon atoms are larger than sodium atoms
SO3 is an empirical formula for which of the following?
Answers
Answer:
what are the options
Explanation:
there are no options
SO₃ is an empirical formula for S₂O₆ and the correct option is option C.
The empirical formula of a compound represents the simplest, most reduced ratio of the atoms present in the compound. It shows the relative number of atoms of each element in the compound, without indicating the actual number of atoms. In other words, it gives the simplest whole number ratio of the elements in a compound.
To determine the empirical formula, the relative amounts of each element in the compound are expressed in terms of moles or mass. Then, the ratios of the moles or masses are simplified to their lowest whole number values.
Thus, the ideal selection is option C.
Learn more about Empirical formula, here:
https://brainly.com/question/32125056
#SPJ6
The complete question is -
SO₃ is an empirical formula for which of the following?
A. S₄O₂
B. SO₄
C. S₂O₆
D. S₂O₃
PLEASE HURRY
Which substance loses electrons in a chemical reaction?
A.the one that is oxidized, which is the oxidizing agent
B.the one that is reduced, which is the reducing agent
C.the one that is oxidized, which is the reducing agent
D.the one that is reduced, which is the oxidizing agent
Answers
Answer:
4) the one that is reduced, which is the oxidizing agent
Explanation:
An oxidizing agent is one that causes oxidation by gaining electrons from another atom/molecule.
Answer:
c
Explanation:
Find ∆H for the reactions given below using Hess's Law. Show your work on a separate
sheet of paper.
1. Fe₂O3(s) + 2Al(s) →Al₂O3(s) + 2Fe(s)
a. 2Al(s) + 3/20₂(g) → Al₂O3(s); ∆H = -1675.7 kJ
b. 2Fe(s) + 3/20₂(g) →Fe₂O3(s); AH = -824.2 kJ
Answers
To find ∆H for the reaction Fe₂O₃(s) + 2Al(s) → Al₂O₃(s) + 2Fe(s) using Hess's Law, we need to use the given reactions (a) and (b) and manipulate them to obtain the desired overall reaction.
Given:
a. 2Al(s) + 3/2O₂(g) → Al₂O₃(s); ∆H = -1675.7 kJ
b. 2Fe(s) + 3/2O₂(g) → Fe₂O₃(s); ∆H = -824.2 kJ
To match the stoichiometry of the desired reaction, we need to multiply reaction (a) by 2 and reaction (b) by 2:
2a. 4Al(s) + 3O₂(g) → 2Al₂O₃(s); ∆H = 2*(-1675.7 kJ) = -3351.4 kJ
2b. 4Fe(s) + 3O₂(g) → 2Fe₂O₃(s); ∆H = 2*(-824.2 kJ) = -1648.4 kJ
Now, we can add these manipulated reactions to obtain the desired overall reaction:
4Al(s) + 3O₂(g) + 4Fe(s) + 3O₂(g) → 2Al₂O₃(s) + 2Fe₂O₃(s)
To find the ∆H for the overall reaction, we add the ∆H values of the manipulated reactions:
∆H = (-3351.4 kJ) + (-1648.4 kJ) = -4999.8 kJ
Therefore, the ∆H for the reaction Fe₂O₃(s) + 2Al(s) → Al₂O₃(s) + 2Fe(s) using Hess's Law is -4999.8 kJ.
\(\huge{\mathfrak{\colorbox{black}{\textcolor{lime}{I\:hope\:this\:helps\:!\:\:}}}}\)
♥️ \(\large{\textcolor{red}{\underline{\mathcal{SUMIT\:\:ROY\:\:(:\:\:}}}}\)
At what rate does magma deep below Earth's surface cool? What size of crystal results from magma cooling deep below Earth's surface?
O A. Slowly, large crystals form
B Quickly, large crystals form
C. Slowly, small crystals form
D. Quickly, small crystals form
Answers
Answer:
i think it is A
Explanation:
i got this answer from a good friend
The magma deep below Earth's surface cool slowly.Large crystals form results from magma cooling deep below Earth's surface. Therefore, option A is correct.
What is magma ?Magma is the molten or semi-molten natural material that gives rise to all igneous rocks. Magma can be found beneath the Earth's surface, and evidence of magmatism has been discovered on other terrestrial planets and natural satellites.
When magma slowly cools deep within the crust, the resulting rock is known as intrusive or plutonic. Because of the slow cooling, crystals can grow large, giving intrusive igneous rock a coarse-grained or phaneritic texture. Individual crystals in phaneritic texture can be seen with the eye.
Intrusive igneous rocks form when magma slowly cools and solidifies beneath the Earth's surface. Large, well-formed crystals are found in intrusive igneous rocks such as granite, gabbro, diorite, and dunite.
Thus, option A is correct.
To learn more about the magma, follow the link;
https://brainly.com/question/16940653
#SPJ6
How many molecules are there in 24 grams of FeF_3?
Answers
1.2 × 10²³ molecules are there in 24 grams of FeF₃ molecule. 1 mole is equal to 6.023 × 10²³ particles.
What do you mean by mole ?A mole is a important unit of measurement that chemists use in calculation. 1 mole is exactly equal to the 6.023 × 10²³ particles.
The term mole is defined as the amount of substance containing 6.023 × 10²³ molecules.
First multiply the number of moles supplied by Avogadro's Number to detect how many molecules there are.
Moles are determined from the molecular weight.
For FeF₃ molecule,
55.8 + 3⋅19 = 116g/mole
24 gram / 116g/mole
= 0.207 moles of FeF₃
= 0.207 moles × 6.023 × 10²³
= 1.2 × 10²³ molecules
Thus, in 24 grams of FeF₃ molecule 1.2 × 10²³ molecules are present.
To learn more about the mole, follow the link;
https://brainly.com/question/7805384
#SPJ9
what is the molarity of an ethanol (c2h6o) solution that was prepared by dissolving 50.500 g of ethanol into water to a final volume of 750.0 ml? assume the solution has a density of 1.20 g/ml.
Answers
The molarity of the ethanol is 11.29M .
What is molarity ?
Molar concentration, also known as molarity, amount concentration, or substance concentration, is a unit used to describe the amount of a substance in a solution expressed as a percentage of its volume. The number of moles per litre, denoted by the unit symbol mol/L or mol/dm3 in SI units, is the most widely used unit for molarity in chemistry. One mol/L of a solution's concentration is referred to as one molar, or 1 M.
The mass of one mole of ethanol is 46.07 g/mol.
The number of mole in 50.500 g ethanol is 50.500/46.07 = 1.096158 mole.
We know that,
Mass = Density × Volume.
The final volume of the solution 750.0ml and the density of the solution is 1.20g/ml.
The mass of the solution is 1.20g/ml × 750.0ml = 900 gm.
The mass of water is (900-50.500) = 849.5 gm.
So the term molarity is defined as:
One of the most common units of concentration, expressed in M. It is defined as no moles of solute in 1 liter of solution.
The molarity of the solution is:
(1.09615 × 1000)/ 849.5 = 1.29M (approx.).
To know more about molarity, click the given link ;
https://brainly.com/question/26873446
#SPJ4
If you were to fill up a tub with water at the very highest that the water could go, and then sit inside the tub, do you think the water would sink due to your density upon the water, or would you submerge into the water (the water arises above you?)

Answers
Answer:
the water would rise above you and spill out
Explanation:
the water would form around you, because the human body is solid, and would take up some of the volume of the tub. the water then goes up due to the added volume.
What is ∆H for the reaction of sulfur dioxide with oxygen?
2SO2(g) + O2(g) → 2SO3(g)
(∆Hf0 SO2(g) = –296.8 kJ/mol; ∆Hf0 SO3(g) = –395.7 kJ/mol)
A.)–98.9 kJ
B.)–197.8 kJ
C.)1.385 × 103 kJ
D.)197.8 kJ
Answers
Answer:
B.) –197.8 kJ
Balanced equation : 2SO₂(g) + O₂(g) → 2SO₃(g)
Given:
SO₂(g) = –296.8 kJ/molSO₃(g) = –395.7 kJ/mol================================================================
products : 2 ( –296.8 ) + O₂ = -593.6 kJ/mol + O₂reactant : 2 ( –395.7 ) = -791.4 kJ/molproducts energy = reactant energy
-593.6 + O₂ = -791.4
O₂ = -791.4 - ( -593.6)
O₂ = –197.8 kJ
A teacher divides her class into groups and assigns each
group the task of measuring the mass of the same object
three times. The teacher already knows that the mass of
the object is 25 g.
Based on the results each group records, which group makes the most
precise measurements of the object?
O
A. Group C: 32.1 g, 35.0 g, 25.0 g
O
B. Group B: 25.5 g, 25.0 g, 24.8 g
O
C. Group A: 28.5g, 28.4 g, 28.5 g
O D. Group D: 20.0 g, 25.0 g, 30.09
Answers
Answer:
Group C
Explanation:
Precision is how close the values are to each other
HELP PLS!! WILL GIVE BRAINLIEST What is a catalyst? A a substance that slows a reaction down without being used up itself in a reaction. B a substance that is used up while speeding up a reaction. C a reactant that is added to speed up a reaction. D a substance that increases the rate of a reaction without being used up itself in a reaction.
Answers
Answer:
D a substance that increases the rate of a reaction without being used up itself in a reaction.
Explanation:
Examples are
ammonia synthesis ==> iron
sulfuric acid manufacture ==> nitrogen(II) oxide, platinum
cracking of petroleum==> zeolites
hydrogenation of unsaturated hydrocarbons ==> nickel, platinum, or palladium
I hope it helps
Answer:
Hey there!
The correct answer would be D. "a substance that increases the rate of a reaction without being used up itself in a reaction."
Hope this helps :)
Charge carriers in a metal are electrons rather than protons because electrons are?
Answers
Charge carriers in a metal are electrons rather than protons because electrons are free to move and weakly restrained .
What are Charge carriers?A particle or quasiparticle that is free to move and carrying an electric charge is known as a charge carrier. This definition specifically refers to particles that carry electric charges in electrical conductors.Examples include ions, holes, and electrons. The subject matter is most frequently employed in solid state physics.The charge carriers that convey positive charge while travelling from one location to another are the positive charge carriers, such as holes. The voids that migrate from one location in the valence band to another are referred to as holes.Protons carry a different kind of charge than electrons, which both carry the same amount of charge.Learn more about charge here:
https://brainly.com/question/13871705
#SPJ4
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
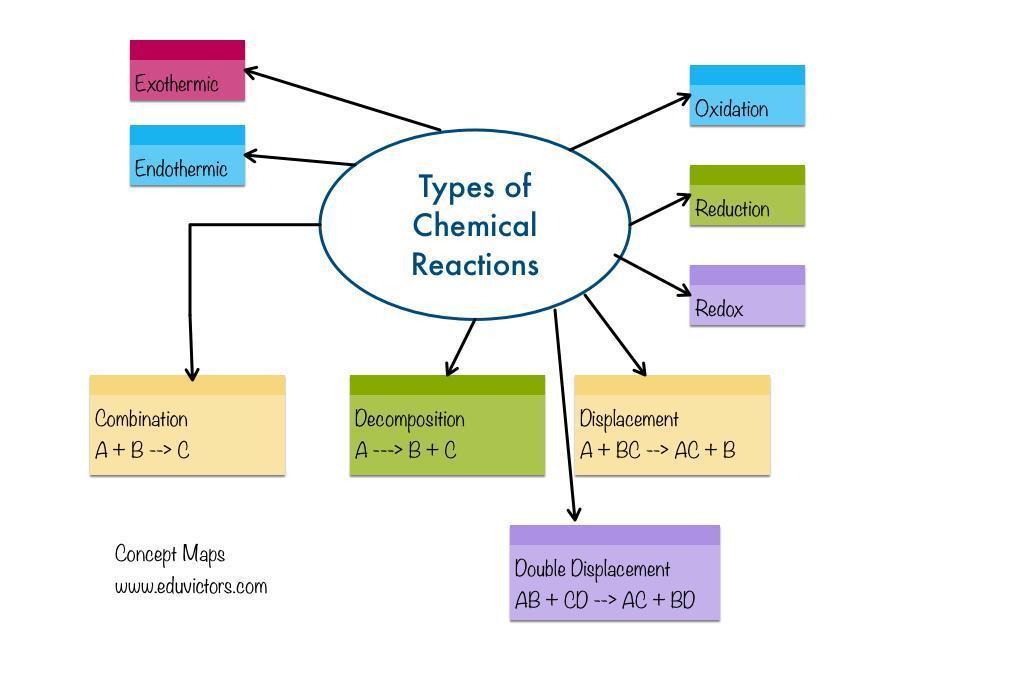
1
Which of the following is an example of a body fossil?
А
A perfect mold of a dinosaur bone
B
A carbon print left by a leaf
C
The skull of a mammoth that fell into a tar pit
D
A dinosaur footprint
Answers
Answer:
A perfect mold of a dinosaur bone
If these students repeated their experiment usin a longer table what difference would they observe? Explain your answer
Answers
given your understanding of the basic metal-cutting process, what are the important physical and chemical properties of a cutting tool?
Answers
The following qualities must be present in a cutting tool:
1. Hot Hardness
2. Hardiness
3. Resistance to Wear
4. Chemical Inertness or Stability
5. Resistance to Shock
6. Reduced Friction
7. Affable Price.
Heat is produced during the cutting of metal. Nearly 600°C to 1800°C is a high raised temperature, and the tool material must be able to keep its hardness, wear resistance, and strength at this temperature. The fluctuation in hardness of several tool materials with an increase in temperature .
The fundamental principle of all metal cutting operations is to gradually push a cutting tool with one or more cutting blades through the surplus material on the work piece. While power is applied, a machine tool and its accessories securely hold the work piece and the tool.
To learn more about cutting tool, click here .
https://brainly.com/question/30024725
#SPJ4
How many outermost electrons do lithium and potassium have
Answers
Answer:
Lithium = 3 electrons (Neutral atom)
Potassium = 19 electrons
Correct Question:-
How many outermost shell/orbit contains electrons do lithium and potassium have.
Lithium Atomic number - 3
Ans :- 1 electron
(K -2 , L - 1)
potassium Atomic number - 19
Ans :- 1 electron
(K - 2 , L - 8 , M - 8, N -1)
Copper(II) sulfate is an example of a substance that dissolves in water. Copper(II) sulfate is an ionic compound with the chemical formula CuSO4 and when it is added to water it dissociates into Cu2+ and SO42− ions. Students used both copper sulfate crystals and copper sulfate powder in this experiment on the rate of dissolving. Choose one statement that represents a valid conclusion based on the student data?
A) The smaller the solute particles the more energy they have. Increased energy results in shorted dissolving time.
B) A given quantity of solute dissolves faster when it is ground into small particles because more surface area is exposed.
C) As the water temperature increased, the copper sulfate crystals dissolved faster but there was little change for the powdered form.
D) Agitation of the powder allows fresh solvent molecules to continually be in contact with the solute

Answers
As the water temperature increased, the copper sulfate crystals dissolved faster but there was little change for the powdered form.
Does increase in temperature make solids to dissolve faster?Increasing the temperature of a solvent can increase the rate at which a solid dissolves. This is because an increase in temperature causes the solvent molecules to move more rapidly, which in turn increases their kinetic energy and ability to interact with the solute particles.
As a result, the solute particles are more likely to break free from their crystal lattice and become surrounded by the solvent molecules.
Learn more about dissolution:https://brainly.com/question/23851972
#SPJ1
Answer:
A given quantity of solute dissolves faster when it is ground into small particles because more surface area is exposed.
Explanation:
The more surface area of a solute that is exposed, the faster the solute will dissolve. Like sugar for an example, sugar cubes take longer to dissolve as compared to regular ground up sugar.
And I took the test and got this right!
What reagent or reagents is reuired for the conversion of yclohexene to cycloexane?
Answers
Reagents required for conversion of clohexene to cycloexane is H2,Pt.
Functions do reagents serve in chemistry:
A chemical reaction is used to confirm the detection of another substance and is referred to as a "substance or compound that is added to a system in order to bring a chemical reaction or is added to check whether a reaction has occurred or not."
Reagents required for conversion of clohexene to cycloexane is H2,Pt.
Hydrogenation of cyclohexene in presense of Pt catalyst gives cyclohexane .
cyclohexene + H2 -----(Pt)----> cyclohexane .
Pt is acting here as a catalyst.
Learn more about Reagents here:
https://brainly.com/question/28196410
#SPJ4
You are creating ammonia via the Haber process which follows this chemical equation. N₂+3H₂ 2N H₂ You need to calculate how much ammonia you can make with the amount of gases you already have but you have a feeling you might not have enough nitrogen to react with all of your hydrogen and don't want excess hydrogen gas in your ammonia If you have 12 moles of hydrogen gas, how many moles of nitrogen gas will you need to completely react with your hydrogen gas?
Answers
N₂(g) + 3H₂(g) → 2NH₃(g) If we have 12 moles of hydrogen gas, 4.0 moles of nitrogen gas will need to completely react with hydrogen gas.
What do you mean by mole ?The amount of substance of a system which includes as many elementary entities is called as mole.
One mole of any substance is equal to 6.023 × 10²³ units of that substance such as atoms, molecules, or ions. The number 6.023 × 10²³ is called as Avogadro's constant.
In Haber process, N₂ reacts with H₂ and formed NH₃.
The balanced equation for the Haber process is as follows:
N₂(g) + 3H₂(g) → 2NH₃(g)
The stoichiometric ratio of H₂ and N₂ is 3 : 1
Therefore, reacted moles of N₂ = reacted moles of H₂ / 3
So, moles of N₂ = 12.0 mol / 3
= 4.0 mol
Thus, 4.0 moles of N₂ is needed to react with 9.0 moles of H₂.
To learn more about the mole, follow the link;
https://brainly.com/question/26416088
#SPJ9
Identify the key differences between the Articles of Confederation and the U.S. Constitution. Then explain which document created the better system of government for the new nation, and support your response with the differences you have identified.
22 POINTS + WILL RATE BRAINLEST!!!!!
Answers
The key differences between the Articles of Confederation and the U.S Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
The document created the better system of government for the new nation is the US constitution.
The Article of confederation , the state have stronger power than the central power and in the US constitution , the power of central government is stronger than the states. The important development was the establishment of three departments. There are three departments of government that is legislative , executive and judicial.
Thus, The key differences between the Articles of Confederation and the U.S. Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
To learn more about US Constitution here
https://brainly.com/question/29027032
#SPJ1
Discuss 3 factors that affect the solubility of a solid in a liquid.
Answers
Answer:
Explanation:
Temperature: increasing the Temperature increases the solubality
Polarity: increase of polarity increases the soluablity of polar solutes
The larger the molecules of the solute are, the larger is their molecular weight and their size. It is more difficult it is for solvent molecules to surround bigger molecules.
For majority of solid and liquid solutes, pressure does not affect solubility. it is applciaable only for gasesous solute
Hope this helps you
Is H2SO4 + NaOH > Na2SO + H2O balanced
Answers
Answer:
H2SO4 + 2NaOH ----> Na2SO4 + 2H2O
Explanation:
This is balanced equation
I NEED THIS LESS THAN 7 MINUTES IYS 15 POINTS!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! What is the difference between a balanced equation and an unbalanced equation?
All of the answers are correct.
A balanced equation obeys the law of conservation of mass, an unbalanced equation does not. An unbalanced equation shows the conservation of mass.
Balanced equations do not show equal numbers of atoms on each side of the equation.
Answers
Answer:
Balanced equation have equal number of atoms of different elements in the side of reactants and products.
Answer:
- A balanced chemical equation has an equal numbers of atoms of different elements in the side of reactants and products.
- An unbalanced chemical equation has an unequal number of atoms of one or more elements in the reaction.
Explanation:
How many mL of 0. 8291 M NaCl are required to prepare 500. 00 mL of a 0. 0500 M NaCl olution?
Answers
To prepare 500. 00 mL of a 0. 0500 M NaCl Solution of 0. 8291 M NaCl 30.48 ml volume is needed
M1=0.82
V1=?
M2=0.05
V2=500 ml
M1V1=M2V2
0.82×V1=500×0.05
V1=30.48 ml
The area occupied within an object's boundaries determines its volume in three dimensions. On occasion, it is referred to as the object's capacity. The volume of an object can be used to calculate how much is needed to fill it; for instance, how much water is needed to fill a bottle, aquarium, or water tank. A sphere is the most basic and prevalent type of three-dimensional shape. We come across spheres all the time in the form of balls, globes, ornamental lights, oranges, etc.
To know more about volume visit :https://brainly.com/question/24189159
#SPJ4