Why is actual yield less than theoretical.
Answers
Answer:
In an actual reaction, unless circumstances are absolutely perfect (which never occurs), the reactants do not completely react with one another. Theoretical yields are expected yields if every bit of each reactants completely reacts with the others.
Related Questions
What are the graduations on the ruler?
Answers
suppose another girl pulls the heavy crate with 10 units of force in the same direction as the girl, what will be the net force that will act on the object? will the object move?
Answers
Answer: yes the net force will same because there is no object that opposed the force of girl in opposite directions
Explanation:
If the density of a gas is 1.2 g/L at 745 torr and 20 degree celsius, what is its molecular mass?R = 0.0821 L.atm/K.mol
Answers
The question requires us to calculate the molecular mass of a gas, given its density (1.2 g/L) and conditions of pressure (745 torr) and temperature (20°C).
Density is defined as the mass of a compound over its volume. From this definition, we can calculate the molecular mass of the gas knowing the density, as given by the question, and the volume of 1 mol of the gas.
\(\text{density = }\frac{mass}{\text{volume}}\to\text{mass = volume }\times\text{ density}\)We can calculate the volume of a gas considering the equation of Ideal Gases:
\(P\times V=n\times R\times T\)where P is the pressure of the gas, V is its volume, n is the number of moles, R is the constant of gases and T is the temperature.
Note that the constant of gases R was given in units of L.atm/K.mol, while the pressure and temperature were given in Torr and °C, respectively. Thus we need to convert these values to the appropriate units.
Knowing that 1 Torr corresponds to 0.00131579 atm:
1 Torr --------------------- 0.00131579 atm
745 Torr ---------------- x
Solving for x, we have that 745 Torr corresponds to 0.980 atm.
To convert the temperature from Celsius degrees to Kelvin, we must add 273.15:
T = 20 + 273.15 K = 293.15 K
Therefore, the pressure and temperature we'll use in our calculation are 0.980 atm and 293.15 K. Also, since we are calculating the molecular mass, we'll consider 1 mol of gas.
Rearranging the equation of ideal gases to calculate the volume and applying the values to the equation, we'll have:
\(\begin{gathered} P\times V=n\times R\times T\to V=\frac{n\times R\times T}{P} \\ V=\frac{(1mol)\times(0.0821L.atm/K.mol)\times(293.15K)}{(0.980\text{atm)}}=24.6L \end{gathered}\)Therefore, the volume of 1 mol of the gas under the conditions given is 24.6L.
Next, we'll use this value to calculate the molecular mass using the density given by the question:
\(\begin{gathered} \text{mass = volume }\times\text{ density} \\ \text{mass = 24.6L}\times1.2g/L \\ \text{mass = }29.5g/\text{mol} \end{gathered}\)Therefore, the gas given by the question presents 29.5g per mol.
Pascal’s Law states that when pressure is applied from an outside source to a contained fluid, the force is
a. transferred through the liquid in a single direction
b. transferred through the liquid in the opposite direction
c. transferred unequally through the liquid in all directions
d. transferred equally through the liquid in all directions
Answers
Answer:
a
Explanation:
Answer:
a.....................
1. Find the mass of 0.89 mol of CaCl2.
Answers
Answer:
97.9
Explanation:
m= n*M
M CaCl2= 40+ 35.5*2= 110
m= 0.89*110= 97.9
The mass of the given mole of \(CaCl_2\) is required.
The mass of the given compound is 98.78 g.
Molar mass of \(CaCl_2\)
\(M=40.078+35.453\times 2=110.984\ \text{g/mol}\)
n = Number of moles = 0.89 mol
Mass is given by
\(m=Mn\\\Rightarrow m=110.984\times 0.89\\\Rightarrow m=98.78\ \text{g}\)
The mass of the given compound is 98.78 g.
Learn more:
https://brainly.com/question/14896336
https://brainly.com/question/13798430
A reaction conducted in a calorimeter consumed 0.038mol of an unknown substance. The temperature of the 109g of water was increased from 13.2 to 25.7. What is the enthalpy of this reaction in kJ/mol?
Answers
The enthalpy change of the reaction is 148.4 kJ/mol. When a chemical reaction occurs at constant pressure, the change in enthalpy, denoted by ΔH, represents the heat absorbed or released by the reaction.
What is Enthalpy ?
Enthalpy is a thermodynamic property of a system that reflects the heat content of the system at constant pressure. It is denoted by the symbol H and has units of joules (J) or kilojoules (kJ). Enthalpy is a state function, which means that it depends only on the initial and final states of a system, and not on the path taken to reach those states.
To calculate the enthalpy change (ΔH) of the reaction in kJ/mol, we need to use the equation:
ΔH = q / n
where q is the heat absorbed or released by the reaction, n is the amount of substance in moles that underwent the reaction, and ΔH is the enthalpy change in kJ/mol.
We can start by calculating the heat absorbed or released by the reaction using the heat capacity of water and the change in temperature of the water:
q = m × C × ΔT
where m is the mass of water, C is the specific heat capacity of water (4.184 J/g°C), and ΔT is the change in temperature of the water.
Substituting the given values, we get:
q = (109 g) × (4.184 J/g°C) × (25.7°C - 13.2°C) = 5619.9 J
We can convert this to kilojoules (kJ) by dividing by 1000:
q = 5.62 kJ
Next, we need to determine the amount of substance in moles that underwent the reaction. From the given information, we know that 0.038 mol of the unknown substance was consumed.
Finally, we can calculate the enthalpy change of the reaction using the formula:
ΔH = q / n = (5.62 kJ) / (0.038 mol) = 148.4 kJ/mol
Therefore, the enthalpy change of the reaction is 148.4 kJ/mol.
Learn more about Enthalpy from given link
https://brainly.com/question/14047927
#SPJ1
Plz help! The best answer will be marked as brainliest!
Make a funny sentence for flammability and compounds for Magnesium
Here is an example for Mercury, it HAS to for Magnesium
“I love to travel but I do corrode aluminum so we won’t be flying off on adventures in airplanes planes anytime soon”
Answers
Answer:
This is hilarious, every time I ignite, they feed me water, little do they know this heat will defeat, and hydrogen only makes me stronger!
Explanation:
When Hg is burning, it uses the oxygen from H2O and turns it into hydrogen gas, and that contributes to the burning.
which sentence is a scientific statement
Answers
The scientific statement is
D. There is life on some other planet in the universe aside from Earth.
What is scientific statement?A scientific statement is a statement that is based on empirical evidence, logical reasoning, and the scientific method. It is a claim or proposition that can be tested, observed, or measured, and is subject to scrutiny and verification.
Scientific statements are characterized by objectivity, reliance on evidence, and the potential for falsifiability or validation through experiments or further investigation. these statements aim to describe, explain, or predict phenomena in the natural world and are an essential part of scientific inquiry and the advancement of knowledge.
Learn more about scientific statement at
https://brainly.com/question/19894291
#SPJ1
complete question
Which sentence is a scientific statement?
A.
Food cooked in ceramic pots has a better aroma than food cooked in copper pots.
B.
A tall glass of water tastes better with a lemon wedge and ice cubes.
C.
Today, there are more viewers watching baseball than ice hockey on television.
D.
There is life on some other planet in the universe aside from Earth
Which activity is an example of a chemical change?
A) Dissolving table sugar in water.
B) Drops of water forming on a cup of iced tea on a hot day.
C) Melting gold to make jewelry.
D) An old penny rusting.
Answers
Answer:
D.an old penny rusting
Explanation:
A chemical property of iron is that it is capable of combining with oxygen to form iron oxide, the chemical name of rust.
An old penny rusting is an example of chemical change as it is accompanied by formation of a new substance.
What is a chemical change?Chemical changes are defined as changes which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical change.
There are several characteristics of chemical changes like change in color, change in state , change in odor and change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical changes:
1) inorganic changes
2)organic changes
3) biochemical changes
During chemical changes atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical change,here:
https://brainly.com/question/23693316
#SPJ2
If one solution contains 10% salt, and another contains 30% salt, the 30% salt solution is said to be _________ to the 10% salt solution.
Answers
Explanation:
more saturated than solution one
the concentration of hydrogen peroxide _ _ _ _over time.
Answers
Answer:
The concentration of hydrogen peroxide decomposes over time.
Explanation:
The concentration of hydrogen peroxide decomposes over time.
What is hydrogen peroxide?It is a chemical compound whose formula is \(\rm H_2O_2\)
It is a liquid with pale blue and viscous composition.
The uses of hydrogen peroxide are sanitizing, disinfectant, etc.
It decomposes into water and oxygen, by breaking oxygen-oxygen bonding.
Thus, the concentration of hydrogen peroxide decomposes over time.
Learn more about hydrogen peroxide
https://brainly.com/question/18709693
the central part of an atom is what
Answers
Answer:
Nucleus
Explanation:
the nucleus is the central part of an atom where protons and neutrons are held together.the Nucleus is the central core of the atom, around which the electrons (negatively charged subatomic particles) orbit.1. Which term best describes ultraviolet radiation?
a. Harmless
b. Invisible
c. Undetectable d. Beneficial
Answers
What information does an equilibrium constant give about a reaction? A. It tells whether products or reactants are favored at equilibrium. B. It tells how much energy is required for the reaction to happen. C. It tells how long it takes the reaction to reach equilibrium. D. It tells what the rate constant of the reaction is at equilibrium. SUBMIT
Answers
The information does an equilibrium constant give about a reaction is It tells what the rate constant of the reaction is at equilibrium.
What information can we get from the equilibrium constant?The equilibrium constant is a value that relates as one of the species in reactants and in the equilibrium of the product. Where Kc represents the value of the constants of firmness, in a determined temperature, the function of the concentration.
Equilibrium occurs when the rate of the forward reaction is equal to the rate of the reverse reaction. All concentrations of reactants and products are constant at equilibrium.
See more about reaction at brainly.com/question/17434463
#SPJ1
please help pls help 20 points Which of the following is a myth about water conservation? An important part of water conservation is preventing water pollution. Using an electric clothes dryer conserves more water than air-drying clothes. Buying bottled water does not conserve more water than drinking from the tap. A front-loading washing machine uses less water than a top-loading washing machine.
Answers
Answer:
Air-drying clothes conserves more water than using an electric clothes dryer.
An element has an atomic number of 18 and an atomic mass of 40. The number of neutrons in the nucleus of an atom of this element is????
Answers
Answer: 22 neutrons
Explanation: 40 is the mass number = atomic mass = total number of protons and neutrons in atomic nucleus
18 is the number of protons in the nucleus of this atom
Then 40 - 18 = 22 neutrons
and this is Argon
what is the ph of a solution that contains 11.7g of nacl for every 200 ml of solution?
Answers
The solution's pH is 7, and there are 11.7g of sodium chloride in every 200 mL of the mixture.
NaCl weighs 11.7 grams.
58.44 g/mol is the molar mass of sodium chloride.
The moles of sodium chloride are equal to mass/molar mass.
NaCl has a molecular weight of 11.7 / 58.44.
NaCl's moles equal 0.200 mol.
pH equals -log(H+)
pH = 7
In the presence of water, sodium chloride will entirely dissociate into Na+ and Cl, neither of which will undergo hydrolysis. The H3O+ and OH ions in the aqueous solution of sodium chloride cause the water to autoionize entirely. Because of its neutrality, NaCl has no impact on pH. a way to gauge how basic or acidic a material or solution is. On a scale of 0 to 14, pH is measured.
Learn more about pH here:
https://brainly.com/question/30390372
#SPJ4
What conclusion can you draw about the ability of metals to hold on to and attract electrons, as
compared to nonmetals?
Answers
Answer:
Metals react by losing electrons. So, there is high reactivity due to lower attraction. Non-metals react by gaining electrons. So, there is high reactivity due to higher attraction.
Explanation:
Metals react by losing electrons. So, there is high reactivity due to lower attraction. Non-metals react by gaining electrons. So, there is high reactivity due to higher attraction. Also, electrons lost by metals transfer to the nonmetals. It is easier for the metals to lose their valance electrons and form cations rather than gaining electrons.
Metals do not hold on to or attract electrons while nonmetals hold on to or attract electrons.
In the periodic table, metals are found towards the left hand side of the table while nonmetals are found towards the right hand side of the table.
Electron affinity of elements increase from left to right across the period. Electron affinity refers to the ability of elements to attract or hold electrons. This ability increase steadily across the period.
Usually, the electron affinity values of nonmetals are very high showing that they easily hold on to and attract electrons while the electron affinity values of metals is very low showing that they do not easily hold on to and attract electrons.
Learn more: https://brainly.com/question/3964366
How many moles are in 6.95 x 10^24 atoms of sodium?
Answers
Answer:
11.5
Explanation:
1 mole of Na has 6.022 × 10^23
so 6.95 x 10^24 / 6.022 × 10^23 = 11.5 moles
Am I correct?? Help please

Answers
Answer:
I would say you are correct.
Explanation:
Answer:
I believe it'll be A (even though you chose it already).
Explanation:
How many grams are in 1.11 molecules of N2?
Answers
Answer:
15.547437000000029
Explanation:
The abiotic components of an
ecosystem are
B. the animals and plants.
A. not important.
D. invisible.
C. nonliving.
Answers
Answer:
C. nonliving.
Explanation:
PLEASE HELP!!!
Most lakes have rivers flowing out of them, carrying water to the ocean. However, some
lakes, including Great Salt Lake and the Dead Sea, have no outlet. Water flowing into
these lakes leaves only through evaporation. Considering that the water flowing into the
lakes contains many dissolved substances, how does the lack of an outlet affect the
composition of these lakes? What would you expect to happen after many more years
of inflow and evaporation?
Answers
Answer:
I feel like it could lead to flooding
coefficient of 3MgCl2?
Answers
A sample of O2 under 2.31 atm occupies 525 ml at 25.0°C. What volume will it occupy at 10.5 atm at the same temperature?
Answers
At the same temperature, the O2 sample will occupy a volume of approximately 121.9 mL at 10.5 atm.
To solve this problem, you can use the Gas Law formula: P1V1/T1 = P2V2/T2. In this case, the temperature remains constant, so we can simplify the equation to P1V1 = P2V2. Where P1, V1, and T1 are the initial pressure, volume, and temperature, and P2 and V2 are the final pressure and volume
Given:
P1 = 2.31 atm
V1 = 525 mL
P2 = 10.5 atm
Now, we need to find V2:
2.31 atm * 525 mL = 10.5 atm * V2
Solving for V2:
V2 = (2.31 * 525) / 10.5
V2 ≈ 121.9 mL
So, the O2 sample will occupy approximately 121.9 mL at 10.5 atm at the same temperature.
More on gas volume: https://brainly.com/question/23007892
#SPJ11
which of the following is not a hazard associated with methanol?
Answers
Methanol is a hazardous substance that can cause a range of problems if ingested or inhaled.
Some of the common hazards associated with methanol include blindness, respiratory failure, and even death. However, one hazard that is not typically associated with methanol is skin irritation or corrosion. While methanol can be harmful if it comes into contact with the skin, it is not known to cause significant irritation or corrosion in the way that some other chemicals do .Methanol is a toxic substance that is commonly used in industrial applications such as fuel, solvents, and antifreeze. The substance is highly flammable and can cause a range of health problems if ingested or inhaled. Methanol poisoning can lead to symptoms such as dizziness, nausea, vomiting, and even death. However, one hazard that is not commonly associated with methanol is skin irritation or corrosion. Unlike some other hazardous substances, methanol is not known to cause significant skin irritation or corrosion. However, it is important to note that methanol can still be harmful if it comes into contact with the skin. If methanol is spilled on the skin, it should be immediately washed off with soap and water. If methanol is ingested, medical attention should be sought immediately.
methanol is a hazardous substance that can cause a range of health problems if ingested or inhaled. While skin irritation or corrosion is not typically associated with methanol, it is still important to take precautions when handling the substance to avoid any potential harm. If you suspect that you have been exposed to methanol, seek medical attention immediately.
To know more about corrosion visit:
brainly.com/question/30057568
#SPJ11
A 0.5998 g sample of a new compound has been analyzed and found to contain the following masses of elements: carbon, 0.1565 g; hydrogen, 0.02627 g; oxygen, 0.4170 g. Calculate the empirical formula of the compound.
Answers
The empirical formula of the compound is CH2O.
To find the empirical formula of the compound, we need to determine the ratios of the atoms in the compound. To do this, we need to find the number of moles of each element present in the compound.
The molar mass of carbon is 12.01 g/mol, the molar mass of hydrogen is 1.008 g/mol, and the molar mass of oxygen is 16.00 g/mol. The number of moles of carbon in the sample is:0.1565 g × 1 mol/12.01 g ≈ 0.0130 mol. The number of moles of hydrogen in the sample is:0.02627 g × 1 mol/1.008 g ≈ 0.0261 molThe number of moles of oxygen in the sample is:
0.4170 g × 1 mol/16.00 g ≈ 0.0261 mol
Now that we know the number of moles of each element, we can determine the simplest whole number ratio of the elements in the compound. We can do this by dividing each number of moles by the smallest number of moles. In this case, the smallest number of moles is 0.0130 mol.
We get:
Carbon: 0.0130 mol ÷ 0.0130 mol = 1
Hydrogen: 0.0261 mol ÷ 0.0130 mol = 2
Oxygen: 0.0261 mol ÷ 0.0130 mol = 2
for more such questions on empirical formula
https://brainly.com/question/16253224
#SPJ11
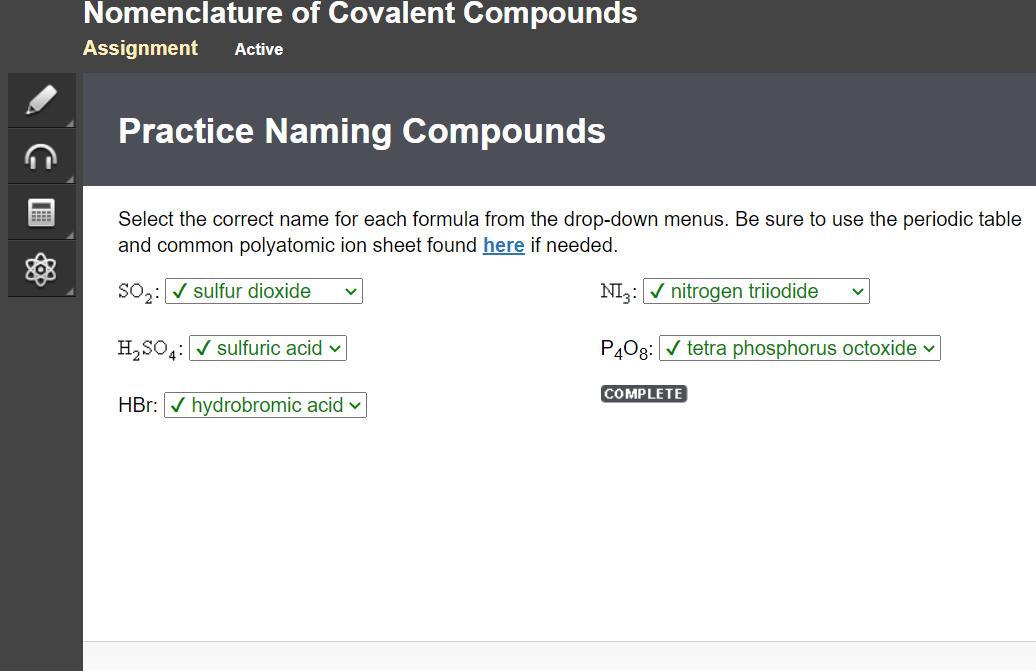
Select the correct name for each formula from the drop-down menus. Be sure to use the periodic table
and common polyatomic ion sheet found here if needed.
Answers
Answer:
here are the answers edge2020
Explanation:

Answer:
Here is 2/5 and the next slide answers too!
Explanation:
Hope it helps!!

Excessive use of chemical fertilizer cause chemical pollution.How?
Answers
Explanation:
Chemical fertilizers raise crop yields, but their heavy usage has hardened the soil, diminished fertility, reinforced pesticides, contaminated air and water, and emitted greenhouse gases, posing health and environmental risks.
a nuclear power plant operates at 40.0 %% efficiency with a continuous production of 1012 mwmw of usable power in 1.00 yearyear and consumes 1.04×106 gg of uranium-235 in this time period. what is the energy in joules released by the fission of a single uranium-235 atom? express your answer numerically in joules per atom.
Answers
Now, we need to take into account the efficiency of the power plant. The efficiency is given as 40.0%, which means that only 40.0% of the total energy input is converted into usable power. We can calculate the total energy input as follows:
Total energy input = Energy produced / EfficiencySubstituting the values, we have:Total energy input = (1012 MW × 1 year × (365 days/year) × (24 hours/day) × (3600 seconds/hour)) / 0.40Now, let's calculate the energy released by the fission of a single uranium-235 atom. First, we need to convert grams of uranium-235 to moles:Moles of uranium-235 = Mass of uranium-235 / Molar mass of uranium-235
Please note that performing the calculations may result in a very large number. It is important to express the answer in scientific notation or using appropriate units such as mega or giga joules to avoid numbers that are too large or small.
To know more about that account visit:
https://brainly.com/question/32040861
#SPJ11