Why does an ultraviolet light cause paint to fade
Answers
Answer:
There are light absorbing color bodies called chromophores that are present in dyes. Ultraviolet rays are one of the causes of fading because they can break down chemical bonds and fade the color in objects.
Related Questions
What is the molecular formula of water vapour?
Answers
Answer:
Water vapor still has the same chemical formula as typical water - H2O - but the water molecules in vapor interact less with one another and are not as structured as they are in water and ice.
Explanation:
The Keq for the interconversion for the two chair conformers of methylcyclohexane at 25 °C is 18. What % of the chair conformers have an axial methyl group?
A) 95 B) 75 C) 50 D) 25 E) 5
Answers
The Keq for the interconversion for the two chair conformers of methylcyclohexane at 25 °C is 18. The % of the chair conformers having an axial methyl group is A) 95%.
If the value of Keq is higher than 1, that means the reaction is favored in the forward direction. If the value of Keq is lower than 1, that means the reaction is favored in the reverse direction.If the value of Keq is equal to 1, that means the reaction is at equilibrium.Methylcyclohexane is an example of the cyclohexane molecule in which there is a substitution of a methyl group on one of the six carbon atoms in the ring structure. The two chair conformers of methylcyclohexane are the axial and equatorial conformers, in which the methyl group is either in an axial or equatorial position, respectively.
The percentage of chair conformers having an axial methyl group can be calculated using the following formula:
% axial conformer = Keq / (1+Keq) × 100
Putting the value of Keq in the formula:
axial conformer = 18 / (1+18) × 100%
axial conformer = 18/19 × 100%
axial conformer = 94.7%
The value of percentage has been rounded off to the nearest whole number, which is 95. Therefore, the answer is (A) 95.
To learn more about equatorial position check the link below-
https://brainly.com/question/32759602
#SPJ11
Do plasmids have an importance beyond the practice of genetic engineering?
Answers
Explanation: Plasmids offer a number of unique characteristics that make genetic engineering much more efficient. Plasmids are a type of non-chromosomal DNA. Integrating DNA into a bacterial or other chromosome is far more complex than simply putting DNA into a cell; plasmids make it easier to transport DNA into a cell by eliminating this step.

How many moles of Silver (1) hydrogen carbonate are produced when 167 g of Tin (Il) hydrogen carbonatecombines with an excess amount of silver (I) dichromate? Use the following balanced equation:1 Sn(HCO3)2 + 1 AgzCr20, ---> 1 SnCr207 + 2 AgHCO3

Answers
Step 1
The reaction:
1 Sn(HCO3)2 + 1 Ag2Cr2O7 => 1 SnCr207 + 2 AgHCO3 (completed and balanced)
-----------------
Step 2
Information provided:
167 g of Tin (Il) hydrogen carbonate, Sn(HCO3)2 => the limiting reactant
The excess = Ag2Cr2O7
-----
Information needed:
The molar mass of Sn(HCO3)2 = 240.7 g/mol (use your periodic table please)
---------------
Step 3
By stoichiometry,
1 mol Sn(HCO3)2 = 240.7 g
Procedure:
1 Sn(HCO3)2 + 1 Ag2Cr2O7 => 1 SnCr207 + 2 AgHCO3
240.7 g Sn(HCO3)2 ---------- 2 x 1 mole AgHCO3
167 g Sn(HCO3)2 ---------- X
X = 167 g Sn(HCO3)2 x 2 x 1 mole AgHCO3/240.7 g Sn(HCO3)2
X = 1.39 moles
Answer: 1.39 moles AgHCO3 are produced
Calculate the mass of 1.25 mol ammonium sulfide, (NH4)2S. *
Answers
Answer:
85g
Explanation:
To convert the moles of a substance to grams we need to know the molar mass of the substance. We, as first, must obtain the molar mass of (NH₄)₂S as follows:
There are 2 atoms of N, 8 of H and 1 of S:
N = 2*14g/mol = 28g/mol
H = 8*1g/mol = 8g/mol
S = 1*32g/mol = 32g/mol
Molar mass: 68g/mol
That means 1 mole of (NH₄)₂S has a mass of 68g.
1.25moles have a mass of:
1.25moles * (68g/mol) =
85gConvert 4.0 moles of N2 gas to liters.
Answers
Convert 4.0 moles of N2 gas to liters.
Answer :-1mole = 22.4 lts of any gas
unitary method :-
so we can say 4 moles = 4 × 22.4 lts of gas
4 moles of N2 gas = 89.6 lts of N2 gas
If 16.4 grams of calcium nitrate is heated as shown in the reaction:
2Ca(NO3)2 -> 2CaO + 4NO2 + O2.
Calculate the volume of nitrogen dioxide produced at STP.
Answers
Answer:
7.2
Explanation:
you first have to find the number of moles of nitrogen dioxide by using the number of moles for calcium nitrate and the mole to mole ratios
number of moles of calcium nitrate=mass/mm
=16.4/102
=0.16g/mol
then you use the mole to mole ratios
2 : 4
0.16: x
2x/2=0.64/2
x=0.32g/moles of nitrogen dioxide
then you use the formula for the volume
v=22.4n
=22.4×0.32
=7.2
I hope this helps
How do reactants change into products during a chemical reaction?
Answers
Answer: During a chemical reaction, reactants are transformed into products through a process known as chemical transformation. This process involves the breaking of chemical bonds between atoms in the reactants and the formation of new bonds between atoms to form the products. The key to this transformation is the rearrangement of the electrons in the atoms involved.
In a chemical reaction, the atoms in the reactants are rearranged to form new chemical compounds, the products. This rearrangement is often accompanied by the release or absorption of energy, leading to the formation of new chemical bonds between atoms. The energy changes that occur during a chemical reaction are closely linked to the chemical changes, and are often responsible for driving the reaction forward.
A chemical reaction can occur through several different mechanisms, but all involve the breaking of existing bonds between atoms and the formation of new bonds to form the products.
Explanation:
What is the electron configuration of the element in period 2 that has 5 valence electrons (valence electrons are the electrons in the outermost shell) *
Answers
Answer:
Nitrogen
Explanation:
N= is the symbol
electronic configuration
1s2 2s2 2p3
Please Help ASAP, any suggestions help

Answers
The correct answer is C. -0.76 V. the potential for this reduction half-reaction is -0.76 V relative to the standard hydrogen electrode.
The correct answer is C. -0.76 V.
The standard reduction potential, denoted as E°, is a measure of the tendency of a species to gain electrons and undergo reduction in a redox reaction. It is expressed in volts (V) and represents the potential difference between the reduction half-reaction and the standard hydrogen electrode (SHE), which is assigned a potential of 0 V.
In the given half-reaction:
Mg2+(aq) + 2e- → Mg(s)
The species undergoing reduction is Mg2+(aq), and it is being reduced to Mg(s) by gaining 2 electrons.
To find the standard reduction potential for this half-reaction, we can refer to standard reduction potential tables. These tables provide a reference for various half-reactions with respect to the standard hydrogen electrode.
In the table, the standard reduction potential for the Mg2+(aq) + 2e- → Mg(s) half-reaction is listed as -0.76 V. This means that Mg2+ has a tendency to be reduced, and the potential for this reduction half-reaction is -0.76 V relative to the standard hydrogen electrode.
Therefore, the correct answer is C. -0.76 V.
for more such question on hydrogen visit
https://brainly.com/question/24433860
#SPJ8
how many atoms are there in 2 moles of oxygen molecules?
Answers
There are 12.044 × 10^23 oxygen molecule present in 2mole of oxygen molecule.
According to the context, the word may as well as may not encompass ions that meet this requirement. A molecule is a collection of two as well as more atoms linked together by the attractive forces described as chemical bonds.
The lowest unit into which a substance could be divided while still being the same substance would be a molecule. It is composed of two as well as more atoms which are chemically bonded to one another.
1 mole of oxygen = 1 O2 molecule
2 mole = 2 O2 molecule = 2 × 6.022 × 10^23 molecule = 12.044 × 10^23 molecule
Thus, 2 mole of oxygen will have 12.044 × 10^23 molecule
Tp know more about molecule
https://brainly.com/question/19922822
#SPJ4
What name would you give a compound consisting of two bromine atoms and one calcium atom?.
Answers
Calcium bromide give a compound consisting of two bromine atoms and one calcium atom.
What is atom?
An atom is a particle of the matter that uniquely defined as a chemical element. An atom is the consists of a central nucleus that is the surrounded by one or more negatively charged of electrons. The nucleus is the positively charged and the contains one or more of relatively heavy particles known as protons and neutrons. Sol-Atomic radius of an atom is defined as the total distance from the nucleus to the outermost shell of the atom. Sodium and magnesium are located in the same era, with magnesium to the right of sodium. They both have the same number of primary shells.
To know more about atom click -
https://brainly.com/question/6258301
#SPJ4
14.
Consider the following balanced chemical equation:
P205
3 H2O → 2 H3PO4
Calculate the mass of hydrogen phosphate, H3PO, that will be produced from 105.9 g of P,Os.
Answers
Answer:
Mass = 72.52 g
Explanation:
Given data:
Mass of hydrogen phosphate produced = ?
Mass of P₂O₅ react = 105.9 g
Solution:
Chemical equation:
P₂O₅ + 3H₂O → 2H₃PO₄
Number of moles of P₂O₅:
Number of moles = mass/molar mass
Molar mass of P₂O₅ 283.9 g/mol
Number of moles = 105.9 g/ 283.9 g/mol
Number of moles = 0.37 mol
Now we will compare the moles of P₂O₅ with H₃PO₄ from balance chemical equation.
P₂O₅ : H₃PO₄
1 : 2
0.37 : 2/1×0.37= 0.74 mol
Mass of H₃PO₄ :
Mass = number of moles × molar mass
Mass = 0.74 mol × 98 g/mol
Mass = 72.52 g
2. Metals react with water and release hydrogen gas. Explain why non-metals do not release hydrogen gas when reacted with water.
Answers
Answer:
Its because non-metals are unable to break the bond between the H and O ion and cannot reduce hydrogen by donating electrons
e structure of (E)-3-phenyl-2-propenal in the window below. • Consider EIZ stereochemistry of alkenes. • In cases where there is more than one answer, just draw one
Answers
The structure of (E)-3-phenyl-2-propenal can be represented as a molecule with a phenyl group attached to the second carbon atom of a propenal chain, where the double bond is in the E-configuration.
The EIZ stereochemistry of alkenes determines the placement of substituents on the double bond based on their relative position to one another. In this case, the phenyl group is on the opposite side of the double bond as the two methyl groups, giving it an E-configuration. The molecule's name provides some important information about its structure. The prefix (E) indicates that the double bond has an E-configuration, meaning the substituents on either side of the double bond are on opposite sides. The 3-phenyl indicates that the phenyl group is attached to the third carbon atom of the propenal chain, while the 2-propenal specifies that the chain has two carbon atoms and an aldehyde group. By using these naming conventions and understanding the EIZ stereochemistry of alkenes, we can accurately represent the structure of (E)-3-phenyl-2-propenal.
Learn more about phenyl group here: brainly.com/question/29448813
#SPJ11
which of the following would most likely reduce the concentration of ground-level ozone in the air of a city?
Answers
Reducing the number of vehicles on the road or promoting the use of public transportation would most likely reduce the concentration of ground-level ozone in the air of a city.
Ozone is a gas that typically exists in the stratosphere, shielding us from sun radiation. It can, however, form on the ground level in some situations.
Existence of nitrogen oxides and volatile organic compounds (VOCs), both produced in factories, industries, and transportation, as well as their reaction catalysed by heat and light, are prerequisites for this to occur.
Due to a lack of heat and light from the Sun on dark, chilly, and gloomy days, the creation of ozone will be reduced.
Additionally, this will allow nitrogen dioxide to accumulate for the same reason, increasing concentration.
Instead of being released into the atmosphere directly, ground-level ozone or "bad" ozone is produced via sunlight-induced chemical interactions between nitrogen oxides (NOx) and volatile organic compounds (VOC).
Learn more about ground-level ozone here
https://brainly.com/question/14804816
#SPJ11
Part C
Bends in rivers are called meanders. Meanders in rivers tend to bend more sharply over time due to uneven erosion on the shoreline at the locations where the river bends. Use your model to investigate this phenomenon. Is there more erosion on the inside or the outside of the curve in the riverbed as water flows through it? Draw an image of your river, and label the location along each curve where the most erosion seems to occur.
Answers
Based on the principles of fluid mechanics and erosion, the water in the river flows faster on the outside of the bend and slower on the inside.
What is the about?Meandering rivers are formed due to the lateral erosion of the riverbank, which creates a curvy path for the water to flow. This is caused by the differences in water velocity between the outside and inside bends of the river.
The above difference in velocity causes the water on the outside to exert more erosive force on the bank, leading to more erosion on the outside of the curve. The slower-moving water on the inside, by contrast, tends to deposit sediment, which can result in the formation of sandbars and other features.
Therefore, it is likely that there is more erosion on the outside of the curve in the riverbed as water flows through it.
Read more about erosion here:
https://brainly.com/question/17905503
#SPJ1
Is primarily responsible for acid rain in the northeast United States
answer choices
O Uranium-238
O Coal
O Natural gas
O Oil
OSolar
Answers
Coal is primarily responsible for the acid rain in the northeast United States.
Acid rain will occurs when the gases such as the sulfur oxide and the nitrogen oxides that are released from the burning of the fossil fuels such as the petrol and the coal are released in the atmosphere, and which then dissolve in the rainwater and fall as the acid rain.
These gases are the acid anhydrides as they will dissolve in the water to produce the acids. In the northeast United States, biggest the source of the acid rain producing pollutants will comes from the burning of the coal by the power plants to produce the electricity.
To learn more about acid rain here
https://brainly.com/question/718250
#SPJ4
Explain the law of mass and give an example that demonstrates it.
Answers
Answer:
it helps bring energy to earth
1. Why is the box sitting on the table not moving?
a) No forces are acting on the box
b) The table pushes up the same force that gravity pushes down
c) Gravity is keeping the box down
d) Gravity is pulling down, but the table is in the way
Answers
if m&m are added to the periodic table as a "new" element what would their atomic mass be? a standard bag of m&ms = 1.75 oz and contains about 55 m&ms. (16oz = 453.6 g) put answer in (g/mol)
Answers
Answer: 5.43x10^23 g/mole M&M's
Explanation:
1 bag of M&M's = 1.75oz (1.75oz)*(453.6g/16oz) = 49.61 g
1 bag = 55 M&M's
(49.61 g)/55 M&M = 0.9021 g/MM
1 mole M&M's = 6.023x10^23 M&M's
(6.023x10^23 M&M's)*(0.9021 g/MM's) = 5.43x10^23 g/mole molar mass
Which principle is included in the u.s contitution
Answers
The principle of separation of powers is an important aspect of the U.S. Constitution. This principle divides the government into three distinct branches: the legislative, the executive, and the judicial. The U.S. Constitution includes the principle of separation of powers, which divides the government into three branches.
The principle of separation of powers is included in the U.S. Constitution. This principle divides the government into three separate branches: the legislative, executive, and judicial branches. Each branch has its own unique responsibilities and powers, ensuring that no single branch has too much control over the government. This principle is crucial to maintaining a balance of power and protecting the rights and freedoms of American citizens. In summary, the answer to your question is that the principle of separation of powers is included in the U.S. Constitution, which divides the government into three separate branches.
One key principle found in the document is the separation of powers. The three branches operate independently of each other to ensure that no single entity holds too much power. The legislative branch, consisting of Congress, is responsible for making laws. The executive branch, headed by the President, enforces and implements laws. Lastly, the judicial branch, led by the Supreme Court, interprets laws and determines their constitutionality.
To know more about powers visit :-
https://brainly.com/question/20030736
#SPJ11
in what two ways do minerals form
Answers
Answer:
Minerals can form in three primary ways being precipitation, crystallization from a magma and solid- state transformation by chemical reactions (metamorphism). Mineral Precipitation is when a mineral is formed by crystallization from a solution. Examples include quartz, halite (table salt), calcite, and gypsum.
Answer:
When magma cools slowly, deep below the surface, it has time to form large crystals in regular patterns. The second way that minerals form is through solutions. (A solution is when one substance is dissolved uniformly in a liquid.) When elements and compounds leave the solution, they can crystalize.
Hypothesis: If a material undergoes a
chemical change, then it will not retain its
original properties because a new substance
is formed.
To test the hypothesis above, you will observe the
changes during the experiment.
To do this, you will use these observations to
compare the___
of the
substances
to the__
substances.
of the

Answers
Answer:
I hate to not answer and have you repost this if you could repost it with the choices by clicking the arrow I can figure it out a lot faster and I'll copy and paste to show you that it's right
Explanation:
I'm good with history biology sum math so if you want to do what I asked and reposted I can give you the answers and I will show that they are correct I won't just guess like some people do just to get points cuz I don't care about points I just get on here to help people
Answer: The answer for the blanks is initial appearance and than final appearance.
Explanation:
write the balance molecular and net ionic equationf for the reaction between almunimum metal and silver nitrate. identify the oxidation and reduction half-reactions
Answers
The balanced chemical reaction is Al + 3AgNO3 → Al (NO3)3 + 3Ag.
A balanced chemical equation though has the identical number of atoms from every type inside the reaction on both the reactant chemical equation output sides. In a balanced chemical equation, both the mass and the change were equal.
An organic organization may adjust to changes in its surroundings very easily. It is distinguished by low complexity, low centralization, as well as low formalization. A mechanistic organization, on the other hand, is distinguished by great complexity, high centralization, as well as high formalization.
Thus, the balanced chemical reaction will be Al + 3AgNO3 → Al (NO3)3 + 3Ag.
To know more about balanced chemical reaction
https://brainly.com/question/30230799
#SPJ4
Put the rules in order, first rule up top.
FIRST RULE
= Balance Oxygen
= Write down the elements
= Balance Hydrogen
= Balance non-metals
Balance metals
Answers
Answer:
Write down the elementsBalance MetalsBalance Non- metalsBalance OxygenBalance Hydrogen.

a good price to pay for good help
Answers
Answer:
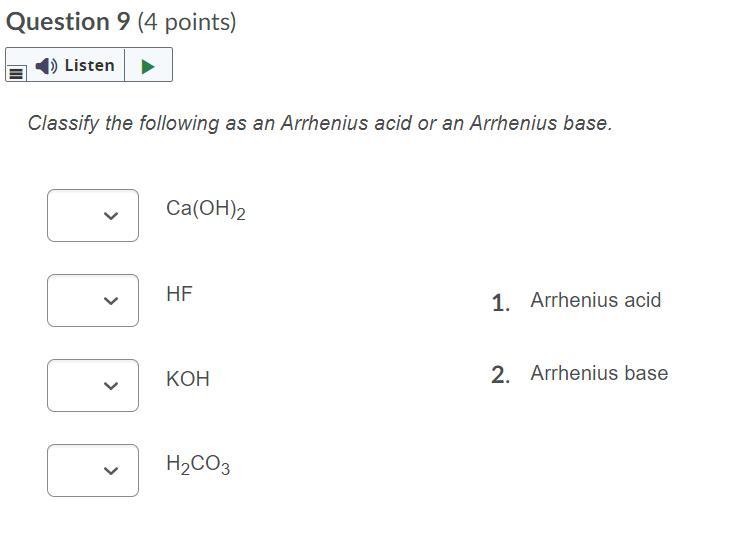
Ca(OH)2 ---> Arrhenius baseHF ----> Arrhenius acid KOH ---> Arrhenius base H2CO3 ---> Arrhenius acidWhat is the mass of 7.00 moles of H2O2
Answers
Answer:
0.206 mol H₂O₂
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:
Step 1: Define
7.00 moles H₂O₂
Step 2: Identify Conversions
Molar Mass of H - 1.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H₂O₂ - 2(1.01) + 2(16.00) = 34.02 g/mol
Step 3: Convert
\(7.00 \ g \ H_2O_2(\frac{1 \ mol \ H_2O_2}{34.02 \ g \ H_2O_2} )\) = 0.205761 mol H₂O₂
Step 4: Check
We are given 3 sig figs. Follow sig fig rules and round.
0.205761 mol H₂O₂ ≈ 0.206 mol H₂O₂
Answer:
238.14
Explanation:
2 H + 2O
2(1.01) + 2(16.00) = 34.02
34.02*7 = 238.14
238.14
is electromagnetic energy and metals physical or chemical change?
Answers
Answer:
Chemical changes release a form of energy called electromagnetic energy, which travels through space as waves.
Is Ni(NO3)3 ionic or covalent and why
Answers
The Ni ( NO₃ )₃ is an anhydrous covalent compound. Ni ( NO₃ )₃ is prepared by the reaction of red fuming nitric acid and nickel nitrate hemihydrate.
What is covalent compound ?An electron exchange that results in the formation of electron pairs between atoms is known as a covalent bond. Bonding pairs or sharing pairs are the names given to these electron pairs. Covalent bonding is the stable equilibrium of the attractive and repulsive forces between atoms when they share electrons.
At room temperature, covalent compounds can be found in all three of their physical states and often have low boiling and melting temperatures. Because covalent compounds lack charged particles with the ability to carry electrons, they do not conduct electricity.
Two atoms sharing a pair of electrons make a covalent connection. Because the electron pair is drawn to both nuclei, the atoms are kept together.
Thus, The Ni ( NO₃ )₃ is an anhydrous covalent compound.
To learn more about covalent compound, follow the link;
https://brainly.com/question/21505413
#SPJ2