why do we use methyl benzoate
Answers
Methyl benzoate is a commonly used chemical compound in various industries, including fragrance, flavor, and pharmaceuticals. It is an ester that is produced by the reaction of benzoic acid and methanol.
Methyl benzoate is used as a fragrance ingredient in various products such as perfumes, colognes, and air fresheners due to its pleasant smell. It is also used as a flavoring agent in foods and beverages, including fruit-flavored drinks, chewing gum, and baked goods.
In the pharmaceutical industry, methyl benzoate is used as a local anesthetic and as a solvent for certain medications. It is also used in the production of various chemicals, including dyes, plastics, and resins.
Overall, the versatility and usefulness of methyl benzoate make it an essential compound in various industries.
Some of the primary uses of methyl benzoate are:
1. Fragrance and flavoring agent: Methyl benzoate is used as a scent and flavor enhancer due to its pleasant, fruity aroma. It can be found in various products, including perfumes, cosmetic products, and food flavorings.
2. Solvent: It serves as a solvent for different organic reactions, owing to its ability to dissolve a wide range of organic compounds.
3. Pesticide: Methyl benzoate is an effective pesticide and insect repellent, as it has a toxic effect on insects, fungi, and some types of bacteria.
4. Pharmaceutical industry: It is used in the pharmaceutical industry as an intermediate for the synthesis of other compounds, such as drugs and other organic chemicals.
In conclusion, we use methyl benzoate in various industries because of its versatile properties, which include its use as a fragrance and flavoring agent, solvent, pesticide, and a precursor for other compounds in the pharmaceutical industry.
To know more about Methyl benzoate refer here
https://brainly.com/question/10213530
#SPJ11
Related Questions
1. Calculate the contribution to the molar internal energy from vibrations of the bond of surface-adsorbed CO. Assume T=300K ( the vibration of 12C16O in the gas phase and adsorbed onto a surface)
The reduced mass for this surface-adsorbed CO molecule = 1.9*10^-26 kg
The vibration temperature θvib for this surface-adsorbed 12C16O molecule. Assume the CO bond force constant does not change when CO adsorbs to the surface. = 2340 K
The vibrational partition function for surface-adsorbed CO. Assume T=300K = 0.0204
Answers
To calculate the contribution to the molar internal energy from vibrations of the bond of surface-adsorbed CO, we can use the equation:
Uvib = (1/2)RT^2 * (dlnq/dT)
where Uvib is the contribution to the molar internal energy from vibrations, R is the gas constant, T is the temperature (in Kelvin), and q is the vibrational partition function.
Given that the vibrational partition function for surface-adsorbed CO is 0.0204 and the temperature is 300K, we can plug in these values to get:
Uvib = (1/2)(8.314 J/mol*K)(300K)^2 * (dln(0.0204)/dT)
To find dlnq/dT, we can use the relation:
dlnq/dT = θvib/2T^2
where θvib is the vibrational temperature.
Given that the vibrational temperature θvib for surface-adsorbed 12C16O molecule is 2340 K, we can plug in this value to get:
dlnq/dT = (2340 K)/(2*300K^2) = 0.013
Plugging this value back into the original equation, we get:
Uvib = (1/2)(8.314 J/mol*K)(300K)^2 * (0.013) = 68.9 J/mol
Therefore, the contribution to the molar internal energy from vibrations of the bond of surface-adsorbed CO is 68.9 J/mol.
Learn more about internal energy : https://brainly.com/question/11742607
#SPJ11
trace minerals are obtained from plant and animal sources. their bioavailability can be influenced by all except which of the following factors?
Answers
The following are the factors that influence the body's ability to absorb minerals:
The type of minerals the body contains.
supplements that are consumed without food.
Mineral excretion loss.
Nutritional factors and nutrient intake.
the person's state of health.
Which trace mineral form is typically regarded as the most bioavailable?The bioavailability of oxide forms of trace minerals is typically the lowest, while the sulphate salts of these minerals have a bioavailability of 100%. Complete bioavailability of trace minerals is provided by sulphate salts, which improves flock performance.
The body needs zinc (Zn), copper (Cu), selenium (Se), chromium (Cr), cobalt (Co), iodine (I), manganese (Mn), and molybdenum as essential trace elements.
To know more about bioavailability visit:
https://brainly.com/question/29557827
#SPJ1
Calcium hydroxide is sprayed on acidic gas produced by power stations. The acidic gas is sulfuric acid. What is the word equation between calcium hydroxide and sulfuric acid?
Answers
Answer:
wait so dose it acid burn your body I would say hydrogen but I'm probably wrong tell me if I am
A solution contains 1.27×10 −2
M sodium sulfide and 1.35×10 −2
M potassium hydroxide. Solid iron(III) nitrate is added slowly to this mixture. What is the concentration of sulfide ion when hydroxide ion begins to precipitate? [sulfide] =
Answers
To find the concentration of sulfide ion when hydroxide ion begins to precipitate, we need to determine the point at which the reaction between sodium sulfide and iron(III) nitrate produces a precipitate.
This reaction can be represented by the following balanced equation: Na2S(aq) + Fe(NO3)3(aq) → FeS(s) + 2NaNO3(aq) First, let's write the balanced equation for the reaction between potassium hydroxide and iron(III) nitrate:
3KOH(aq) + Fe(NO3)3(aq) → Fe(OH)3(s) + 3KNO3(aq)
From the balanced equation, we can see that for every 3 moles of potassium hydroxide (KOH), we get 1 mole of Fe(OH)3(s) precipitate.
Therefore, when hydroxide ion begins to precipitate, the concentration of sulfide ion will be equal to the concentration of potassium hydroxide. Given that the concentration of sodium sulfide is 1.27×10^(-2) M and the concentration of potassium hydroxide is 1.35×10^(-2) M, the concentration of sulfide ion [S^2-] at the point of precipitation is also 1.35×10^(-2) M. Therefore, the concentration of sulfide ion when hydroxide ion begins to precipitate is 1.35×10^(-2) M.
To know more about concentration visit:
https://brainly.com/question/30862855
#SPJ11
Indicate the direction of polarity of each of the covalent bonds by placing the appropriate delta notation next to each end of the bond.
Answers
The delta notation, indicating the polarity of for the presented covalent bonds are:
δ+ C-O δ-
δ- O-Cl δ+
δ+ O-F δ-
δ+ C-N δ-
δ+ C-Cl δ-
δ- S-H δ+
δ+ S-Cl δ-
How delta notation indicate the polar bonds?
Atoms with varying electronegativities form polar bonds.
The polarity direction is denoted using the delta notation.
A partial negative charge (represented by the symbol -) is acquired by the atom with the higher electronegativity, whereas a partial positive charge (represented by the symbolδ +) is acquired by the atom with the lower electronegativity.
Let's consider the following elements with their electronegativities.
C (2.5)
O (3.5)
Cl (3.0)
F (4.0)
N (3.0)
S (2.5)
H (2.1)
The delta notation for the presented bonds are:
δ+ C-O δ-
δ- O-Cl δ+
δ+ O-F δ-
δ+ C-N δ-
δ+ C-Cl δ-
δ- S-H δ+
δ+ S-Cl δ-
To learn more covalent bonds:
https://brainly.com/question/3447218
#SPJ4
What is the limiting reactant for the reaction below given that you start with 2.50 grams c and 2.50 grams sio2?
Answers
To determine the limiting reactant, we need to compare the moles of each reactant and their stoichiometric coefficients in the balanced equation. The balanced equation for the reaction is:
C + SiO2 -> SiC + CO2
First, we convert the masses of carbon (C) and silicon dioxide (SiO2) to moles using their respective molar masses. The molar mass of carbon (C) is 12.01 g/mol, and the molar mass of silicon dioxide (SiO2) is 60.08 g/mol.
Moles of C = mass of C / molar mass of C = 2.50 g / 12.01 g/mol = 0.208 mol C
Moles of SiO2 = mass of SiO2 / molar mass of SiO2 = 2.50 g / 60.08 g/mol = 0.0416 mol SiO2
Next, we compare the moles of each reactant to their stoichiometric coefficients in the balanced equation:
From the balanced equation: 1 mol C reacts with 1 mol SiO2
Since the ratio of moles of C to moles of SiO2 is 0.208 mol C : 0.0416 mol SiO2, we can see that the mole ratio is 5:1. Therefore, for every 5 moles of carbon, 1 mole of SiO2 is required.
Since we have equal moles of carbon and silicon dioxide (0.208 mol C and 0.0416 mol SiO2), we can see that the limiting reactant is SiO2. It is the limiting reactant because there is not enough SiO2 to react completely with the available carbon.
Learn more about stoichiometric coefficients here:
https://brainly.com/question/32088573
#SPJ11
At what temperature will 110 g of potassium bromide dissolve?
Answers
Depending on the temperature of the solvent, KBr can dissolve in water up to the corresponding solubility limit, which ranges from approximately 53 g/100 mL to 100 g/100 mL.
At what temperature will 110 g of potassium bromide dissolve?The solubility of potassium bromide (KBr) depends on the temperature of the solvent in which it is dissolved. In general, the solubility of most salts, including KBr, tends to increase with an increase in temperature.
However, to provide a specific answer, we need to refer to a solubility chart or data for KBr. Here is an approximate solubility of KBr in water at different temperatures:
At 0°C (32°F), the solubility of KBr in water is approximately 53 g/100 mL.At 20°C (68°F), the solubility of KBr in water is approximately 60 g/100 mL.At 40°C (104°F), the solubility of KBr in water is approximately 70 g/100 mL.At 60°C (140°F), the solubility of KBr in water is approximately 83 g/100 mL.At 80°C (176°F), the solubility of KBr in water is approximately 100 g/100 mL.Learn more about solubility at:
https://brainly.com/question/1884491
#SPJ1
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm? (c= 3.00 x 108 m/s, h=6.63 * 10-34 J·s; NA = 6.022 1023 moles-1Multiple Choice A 2.46 10-4 b. 6.46 x 10-25, c. 12.4 kJ 6.46 d. 10-16 246 kJ
Answers
The correct option is A) 2.46 x 10^-4. To find the energy in joules of a mole of photons associated with visible light of wavelength 486 nm, we can use the formula E=hc/λ, where E is energy, h is Planck's constant, c is the speed of light, λ is the wavelength.
First, we need to convert the wavelength from nanometers to meters, so we have λ = 486 nm * (1 m / 10^9 nm) = 4.86 x 10^-7 m. Then, we can plug in the values for h, c, and λ:
E = (6.63 x 10^-34 J·s) * (3.00 x 10^8 m/s) / (4.86 x 10^-7 m)
E = 4.08 x 10^-19 J
This is the energy of one photon with a wavelength of 486 nm. To find the energy in joules of a mole of photons, we need to multiply by Avogadro's number (NA = 6.022 x 10^23 mol^-1):
E = (4.08 x 10^-19 J/photon) * (6.022 x 10^23 photons/mol)
E = 2.46 x 10^5 J/mol
This is a relatively large amount of energy, which is why visible light can have effects on chemical reactions and biological processes.
To know more about Planck's constant refer here:
https://brainly.com/question/27389304#
#SPJ11
Identify the noble gas that is used by researchers to cool down samples for analysis.
O helium
O radon
O iodine
O oxygen
O neon
Answers
Neon
The noble gas that is used by researchers to cool down samples for analysis is neon.
What is a noble gas?
A noble gas is a group of elements that make up group 18 of the periodic table. Noble gases are nonreactive, nonmetallic chemical elements that have full outer electron shells (thus making them highly stable) and low reactivity. The six noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
What are the uses of neon?
Neon has a number of applications in a variety of fields, some of which are listed below:
Electronics: neon is used in high-voltage indicators, lightning arresters, and electronic equipment as a low-pressure gas.
Neon lamps are used in night glow signs and decorative lighting for homes and businesses. Lasers: Neon gas is used in gas lasers, which are frequently used in medical equipment and research laboratories.
Radiometric dating: Radon-222, a decay product of radium-226, can be used to date rocks and fossils.
Neon is used by researchers to cool down samples for analysis.
Learn more about noble gas
brainly.com/question/20314892
#SPJ11
The color of the ball is red physical or chemical property
Answers
Answer:
A physical property because there haven't been a change yet.
Explanation:
chemical would be like toxicity or acidity
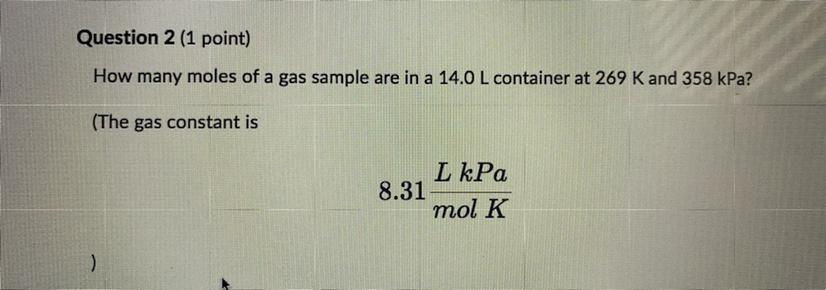
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
An enzyme's specificity can be due to: Group of answer choices amount of substrate available molecular recognition based on structural complementarity the ratio of catalyzed rate to the uncatalyzed rate of reaction. amount of enzyme produced by the cell.
Answers
Answer:
molecular recognition based on structural complementarity
Explanation:
Enzymes work on the basis of a lock and key model. The substrates on which enzymes act are specifically tailored to suit the shape of the enzyme.
As such, the enzyme just fits into the substrate in the same way as the right key fits into a lock.
Hence, molecular recognition based on structural complementarity is the basis for the specificity of enzyme activity.
How many molecules are equal to 3.52 moles of oxygen difluoride?
Answers
Answer:
3.52 moles are equal to 21.2 ×10²³ molecules
Explanation:
Given data:
Number of moles of oxygen fluoride = 3.52 mol
Number of molecules = ?
Solution:
The given problem will solve by using Avogadro number.
1 mole contain 6.022×10²³ molecules
3.52 mol ×6.022×10²³ molecules / 1 mol
21.2 ×10²³ molecules
Thus, 3.52 moles are equal to 21.2 ×10²³ molecules .
What is the pressure of a gas, in millimeters of mercury, in a sample container connected to a mercury-filled, open-ended manometer if the level of mercury in the arm connected to the atmosphere is 3.41 cm higher than the arm connected to the sample container and if atmospheric pressure is 99.54 kPa
Answers
The pressure of the gas in the sample container connected to the manometer is 25.6 mmHg. First, we need to convert the height difference of the mercury levels from centimeters to millimeters. There are 10 mm in 1 cm, so the height difference is 3.41 cm x 10 mm/cm = 34.1 mm.
We need to find the pressure difference between the gas in the sample container and the atmospheric pressure. This can be found by subtracting the height difference of the mercury levels from the atmospheric pressure. 99.54 kPa - (34.1 mm / 760 mmHg/kPa) = 99.54 kPa - 0.045 = 99.495 kPa ,Now we can use the conversion factor of 1 mmHg = 0.133 kPa to convert the pressure difference from kilopascals to millimeters of mercury. 99.495 kPa x (1 mmHg/0.133 kPa) = 748.5 mmHg .
First, convert the atmospheric pressure from kPa to mmHg. 1 kPa is approximately equal to 7.50062 mmHg. So, 99.54 kPa × 7.50062 (mmHg/kPa) ≈ 746.99 mmHg. Convert the difference in mercury levels from cm to mm. 1 cm is equal to 10 mm. So, 3.41 cm × 10 (mm/cm) = 34.1 mm. The level of mercury in the arm connected to the atmosphere is 3.41 cm higher than the arm connected to the sample container, meaning the gas pressure is lower than the atmospheric pressure. Subtract the difference in mercury levels from the atmospheric pressure to find the gas pressure: 746.99 mmHg - 34.1 mm = 726.4 mmHg.
To know more about gas visit :
https://brainly.com/question/18124975
#SPJ11
How many molecules of Acetic Acid (CH3COOH) are in a sample that has a mass of 172.90 g?
Answers
Answer:
17.46× 10²³ molecules
Explanation:
Given data:
Mass of acetic acid = 172.90 g
Number of molecules = ?
Solution:
First of all we will calculate the number of moles of acetic acid :
Number of moles = mass / molar mass
Number of moles = 172.90 g/ 60.1 g/ol
Number of moles = 2.9 mol
Now the given problem will solve by using Avogadro number.
The number 6.022 × 10²³ is called Avogadro number.
1 mole = 6.022 × 10²³ molecules
2.9 mol × 6.022 × 10²³ molecules / 1 mol
17.46× 10²³ molecules
Avogadro number:
It is the number of atoms , ions and molecules in one gram atom of element, one gram molecules of compound and one gram ions of a substance.
the ionization energies (kj/mol) of hydrogen (h) , nitrogen (n) , sodium (na) , and oxygen (o) are 1,312, 1,402, 496, and 1,314, respectively. which element combination is least likely? responses
Answers
The least likely element combination would be hydrogen (H) and sodium (Na) since their ionization energies differ significantly.
To determine the least likely element combination, we need to consider the ionization energies and their relative values. The element combination that is least likely would involve elements with similar or close ionization energies.
Comparing the ionization energies:
1,312 kJ/mol (H) < 1,402 kJ/mol (N) < 1,314 kJ/mol (O) < 496 kJ/mol (Na)
Based on these values, the least likely element combination would be hydrogen (H) and sodium (Na) since their ionization energies differ significantly.
Learn more about element here
https://brainly.com/question/31950312
#SPJ11
GIVING BRAINLIEST The molar mass of AICI3 is 133.34 g/mol. How many molecules of AICI3 are there in 2g?
Answers
Answer:
If I remember correctly, 0.0149991895187944
Explanation:
hope this helps:)
A solution of NaOH has concentration of 1.2M then mass of NaOH in g/dm3 is
Answers
Answer:
A solution of NaOH has concentration 1.2M. Calculate the mass of NaOH in g/dm3 in this solution. = 1.2x 40x 1 = 48 g 3.
The mass of NaOH in the solution having concentration of 1.2 M is 48.00 grams per decimeter cubed (g/dm³).
Concentration refers to the amount of a solute dissolved in a given amount of solvent or solution. It is a measure of how much solute is present in a specific volume or mass of the solution.
Concentration can be expressed in various units, such as molarity (moles of solute per liter of solution), mass percent (mass of solute per 100 grams of solution), parts per million (ppm), and others.
Given:
Concentration of NaOH solution = 1.2 M
The molar mass of NaOH (sodium hydroxide) is:
Na: 22.99 g/mol
O: 16.00 g/mol
H: 1.01 g/mol
Total molar mass of NaOH = 22.99 + 16.00 + 1.01
= 40.00 g/mol
1 mole of NaOH = 40.00 g
1.2 moles of NaOH = 1.2 moles × 40.00 g/mole
= 48.00 g/L
1 L = 1 dm³
So, 48.00 g/L = 48.00 g/dm³
Learn more about Concentration, here:
https://brainly.com/question/30862855
#SPJ3
1. __N2 + __H2-> __NH3
2. __Fe + __HCl-> __H2 + __FeCl3
Answers
Answer:
N2+ 3H2-> 2NH3
2Fe + 6HCl -> 3H2 + 2FeCl3
Which postulate of the kinetic-molecular theory applies to the particles of a liquid?
They do not move freely but vibrate in a stationary position.
They can be compressed, which causes a large change in volume.
They have so much kinetic energy that their intermolecular forces are negligible.
They have enough kinetic energy to easily slide by each other.
Answers
The particles of liquid have enough kinetic energy to easily slide by each other. The correct option is D.
What is kinetic-molecular theory?The kinetic-molecular hypothesis stated matter states and is based on the assumption that matter is made up of tiny particulate that are constantly in motion.
This theory adds to the knowledge of observable properties and behaviors of solids, liquids, and gases.
Because they are densely packed and vibrate in place, solids have the lowest kinetic energy.
Since liquids have higher kinetic energy, the particles slide past each other. Because gases have the most kinetic energy, they float around in the air.
Particles in liquids are very close together and move randomly throughout the container.
Due to the shorter distances between particles, particles move rapidly in all orientations but strike with each other more frequently than in gases.
Thus, the correct option is D.
For more details regarding kinetic molecular theory, visit:
https://brainly.com/question/15013597
#SPJ6
A cell is injected with ion X and placed into a solution. The following potentials are measured inside and outside the cell, yet there is no net flow of X ions. Why?
X must be a positively charged ion, a cation, so that the electrical gradient is enough to keep its concentration higher in the cell at an equilibrium state.
Answers
In order for X to maintain a larger concentration in the cell in an equilibrium state, it must be a positive-charged ion, or a cation.
equilibrium's definition.
A state of equilibrium among opposing factions or activities that can be rigid (like a body acted on by the energy which result is negative) or fluidity (like a chemical process cyclical where the speeds of both sides are equal).
What does the equilibrium of a body mean?
The body's internal energy and motion are unaffected by the time that passes while it is in balance. If anything is in equilibrium, the forces are equalised.
To know more about Equilibrium visit:
https://brainly.com/question/2009610
#SPJ4
A hydrocarbon substituent is called an
Answers
Hydrogen Gas (H₂)
A. 2 atoms of oxygen
B. 2 atoms of hydrogen
C. 1 atom of hydrogen
D. 3 atoms of hydrogen
Answers
to what volume should you dilute 30 ml of a 12 m h2so4 solution to obtain a 0.15 m h2so4 solution?
Answers
The volume will be 2400 ml.
The equation used here will be
M1 × V1 = M2 × V2
M1 = initial concentration
M2 = final concentration
V1 = initial volume
V2 = final volume
So according to the data;
M1 = 12m
M2 = 0.15m
V1 = 30ml
V2 = ?
By putting the values in the equation as follows;
M1 × V1 = M2 × V2
V2 = M1 × V1 / M2
By putting the values given in the question we will solve this question as follows
V2 = 12 × 30 / 0.15
V2 = 360 / 0.15
So the volume used will be;
V2 = 2400 ml
To look more about moles click here
brainly.com/question/26416088
#SPJ4
What is the strongest force that exists between molecules of nitrogen
monoxide (NO)?
Answers
Answer:
Hydrogen Bonding (H-Bonding)
The label on a pressurized can of spray disinfectant warns against heating the can above 130' F . What are the corresponding temperature on the Celsius and Kelvin temperature Celsius?
Answers
Answer:
54.44C and 327.59K
Explanation:
Which of the following reagents would oxidize Ag to Ag+, but not Cl− to Cl2?
Co, Br−, Br2, Co2+, Ca2+
Answers
Br2 is a strong oxidizing agent that can oxidize Ag to Ag+ but it cannot oxidize Cl- to Cl2. This is because the reduction potential of Br2 is higher than that of Ag.
That Br2 has a greater tendency to gain electrons and be reduced. On the other hand, Cl- has a lower reduction potential than Br2, so Br2 cannot oxidize Cl- to Cl2.
Reduction potentials indicate how likely a species is to gain electrons. A higher reduction potential means a species is more likely to gain electrons (be reduced). For a reaction to occur spontaneously, the oxidizing agent (the one being reduced) should have a higher reduction potential than the reducing agent (the one being oxidized). Comparing the standard reduction potentials.
To know more about oxidizing visit:
https://brainly.com/question/13182308
#SPJ11
Liquid nitrogen, which has a boiling point of −195.79°C, is used as a coolant and as a preservative for biological tissues. Is the entropy of nitrogen higher or lower at −200°C than at −190°C? Explain your answer. Liquid nitrogen freezes to a white solid at −210.00°C, with an enthalpy of fusion of 0.71 kJ/mol. What is its entropy of fusion? Is freezing biological tissue in liquid nitrogen an example of a reversible process or an irreversible process?
Answers
Answer:
Explanation:
Entropy is measure of disorder so as we lower the temperature of gas , its entropy decreases .
Hence at - 200°C entropy of nitrogen will be less than that at - 190°C .
At freezing point ,
entropy of fusion = latent heat / freezing temperature
= .71 kJ / ( 273 - 210 )
= 710 / 63 J mol⁻¹ K⁻¹ .
= 11.27 J mol⁻¹ K⁻¹ .
entropy of fusion = 11.27 J mol⁻¹ K⁻¹ .
if there are 12 x 1023 oxygens in a sample of water, how many grams of hydrogen are in the sample?
Answers
I’m sorry that I couldn’t write it, brainly kept saying that I have inappropriate words for some reason.

Carbon monoxide poisoning occurs when carbon monoxide builds up in your bloodstream. When too much carbon monoxide is in the air, your body replaces the oxygen in your red blood cells with carbon monoxide. This can lead to serious tissue damage, or even death. Which 2 different approaches can you use to calculate the number of carbon monoxide molecules in 45 pg of carbon monoxide?
Answers
Two approaches to calculate the number of carbon monoxide molecules in 45 pg are using Avogadro's number or molar mass.
1) Approach using Avogadro's number:
Determine the molar mass of carbon monoxide (CO). The molar mass of carbon is approximately 12.01 g/mol, and the molar mass of oxygen is approximately 16.00 g/mol. Therefore, the molar mass of carbon monoxide (CO) is: 12.01 g/mol (carbon) + 16.00 g/mol (oxygen) = 28.01 g/mol
Convert the given mass of carbon monoxide to moles. 45 pg = 45 x 10⁻¹² g (since 1 pg = 10⁻¹² g) moles = mass / molar mass moles = 45 x 10⁻¹² g / 28.01 g/mol
Calculate the number of molecules using Avogadro's number. Avogadro's number states that there are 6.022 x 10²³ molecules in one mole of a substance. Number of molecules = moles x Avogadro's number of molecules = (45 x 10⁻¹² g / 28.01 g/mol) x (6.022 x 10²³ molecules/mol)
Approach using molar mass
Determine the molar mass of carbon monoxide (CO). The molar mass of carbon monoxide (CO) is calculated as 28.01 g/mol (as explained in Approach 1).
Convert the given mass of carbon monoxide to moles. 45 pg = 45 x 10⁻¹² g (since 1 pg = 10⁻¹² g) moles = mass / molar mass moles = 45 x 10⁻¹² g / 28.01 g/mol
Use stoichiometry to calculate the number of molecules. The balanced chemical equation for carbon monoxide (CO) is: 1 mole of CO = 6.022 x 10²³molecules of CO
Since the stoichiometry is 1:1, the number of molecules is equal to the number of moles. Number of molecules = 45 x 10⁻¹² g / 28.01 g/mol
Both approaches involve using the molar mass of carbon monoxide and Avogadro's number to convert mass to moles and then to molecules.
To learn more about Avogadro's number here
https://brainly.com/question/28812626
#SPJ4