Answers
Answer:
Because they are renewable energy
Both wind and hydroelectric power can also be considered to be forms of solar energy because of the rotation of the sun and the earth consistently generating more winds and electricity.
What is Solar energy?Solar energy may be defined as a type of energy that involves the radiation from the Sun which is capable of producing heat and inducing chemical reactions, or generating electricity. It is also known as solar power.
Wind power such as biomass and hydropower is another form of indirect solar power. Both wind and hydroelectric power are also considered sources of renewable energy.
Hydroelectric power originates from water or the movement of water. It can be seen as a form of solar energy because the sun powers the hydrological cycle that is responsible for the synthesis of water.
Therefore, the rotation of the sun and the earth consistently generates more winds and electricity. So, both wind and hydroelectric power can also be considered to be forms of solar energy.
To learn more about Solar energy, refer to the link:
https://brainly.com/question/17711999
#SPJ2
Related Questions
in a polymer exchange membrane fuel cell h2 and o2 are combined to give water half cell reactions and overall reaction
Answers
This reaction takes place at the anode and cathode of the fuel cell, with hydrogen (H2) being oxidized at the anode and oxygen (O2) being reduced at the cathode. The flow of electrons (e-) through an external circuit provides the electrical power output of the fuel cell.
The half-cell reactions for hydrogen (H2) and oxygen (O2) in a polymer exchange membrane (PEM) fuel cell are:
Hydrogen half-cell reaction:
2H2(g) -> 4H+(aq) + 4e-
Oxygen half-cell reaction:
O2(g) + 4H+(aq) + 4e- -> 2H2O(l)
The overall reaction in the PEM fuel cell can be obtained by combining the half-cell reactions:
2H2(g) + O2(g) -> 2H2O(l)
Polymer Exchange Membrane (PEM) is a type of membrane used in a variety of applications, particularly in fuel cells and electrolysis. It is made of a thin, ion-conductive film sandwiched between two electrodes. The ion-conductive layer allows for the transfer of ions between the electrodes, while the non-conductive layers serve as a barrier, preventing the mixing of the reactants. PEMs are known for their high proton conductivity, fast ion transport, and stability under extreme conditions.
They are also able to operate at low temperatures, making them well-suited for use in portable and mobile applications. PEMs are typically made of hydrocarbon polymers, such as Nafion, and are typically produced in a thin, flexible sheet. Due to their ability to produce and consume hydrogen, PEMs are an important component in the development of fuel cell technology, which is seen as a promising alternative to traditional energy sources.
To learn more about Polymer exchange membrane (PEM) visit here:
brainly.com/question/29898550
#SPJ4
A plate moves 200 m in 10,000 years. What is its rate in cm/year?
Answers
Answer:
The answer is
2 cm/yearExplanation:
To find the rate in cm/year we must first convert 200 m into cm
1 m = 100 cm
if 1 m = 100 cm
Then 200 m = 200 × 100 = 20 ,000 cm
So the rate is
\( \frac{20000}{10000} \)Reduce the fraction with 10,000
We have the final answer as
2 cm/yearHope this helps you
Answer:
2 CM/year just now got 100 Edge.
Explanation:
Which bone is located between the incus and the inner ear?
cochlea
stapes
incus
malleus
Answers
Answer: The answer is incus
A sentence using the word Compound
Answers
Answer:
The air smelled like a compound of diesel and gasoline fumes.
Which process causes earth surface to warm
Answers
ur welcome ;)
A sample of a gas is in a sealed container. The pressure of the gas is 565 torr , and the temperature is 27 ∘C . If the temperature changes to 71 ∘C with no change in volume or amount of gas, what is the new pressure, P2, of the gas inside the container?
Answers
Answer:
P₂ = 647 torr
Explanation:
Given data:
Initial pressure = 565 torr
Initial temperature = 27°C
Final temperature = 71°C
Final pressure = ?
Solution:
Initial temperature = 27°C (27+273 = 300 K)
Final temperature = 71°C (71+273 = 344 K)
According to Gay-Lussac Law,
The pressure of given amount of a gas is directly proportional to its temperature at constant volume and number of moles.
Mathematical relationship:
P₁/T₁ = P₂/T₂
Now we will put the values in formula:
565 torr / 300K = P₂/344 K
P₂ = 565 torr × 344 K / 300 K
P₂ = 194360 torr. K /293 K
P₂ = 647 torr
Part A
Consider the following neutral electron configurations in which n has a constant value. Which configuration
would belong to the element with the most negative electron affinity, Eea?
View Available Hint(s)
a 2s2
b 2s²2p2
c 2s²2p5
d 2s²2p6
Answers
Answer:
c. 2s2 2p5
Explanation:
2s2 2p5 has 7 valence electrons and only needs one electron to complete the octet. This element will be the most electronegative.
Jewelry and tableware are sometimes made of sterling silver. Sterling silver is 92.5% silver (Ag) and 7.5% copper (Cu). If you wanted to make 30 grams of sterling silver for a ring, how many grams of silver and copper would you need to start with?
Amount of sterling silver?
Amount of copper?
total grams of alloys needed?
Answers
Answer:
12.33
Explanation:
92.5/7.5= 12.33
30g of the sterling silver ring contains, 27.75 g of silver and 2.25 g of copper.
What is cross multiplication?Cross multiply fractions by multiplying one fraction's denominator by the other fraction's numerator and then comparing the two values. The larger fraction is the one with the higher value.
100g of sterling silver contains 92.5g of silver and 7.5g of copper.percentage of element = amount of the element present in 100 g of materialTherefore,
100g sterling silver = 92.5g silver30g sterling silver = X g silver( cross multiplication),
100 × X = 92.5 × 30
X = 92.5 × 30/ 100
X= 27.75g silver100g sterling silver = 7.5g copper
30g sterling silver= Y g copper
(cross multiply)
100 × Y = 7.5 × 30
Y= 7.5 × 30 / 100
Y= 2.25g copperX (silver)= 27.75 g in 30 g of sterling silverY (copper)= 2.25 g in 30 g of sterling silvertherefore, 30g of sterling silver ring contains, 27.75 g of silver and 2.25 g of copper.
To learn motre about cross multiplication, refer to:
https://brainly.com/question/28839233
#SPJ2
One of the most important uses for Grignard reagents is their addition to carbonyl compounds to give new carbon-carbon bonds.
a. True
b. False
Answers
Answer:
a. True
Explanation:
Hello there!
In this case, as Grignard reagents are widely used in organic-synthesis procedures, since they are compelling nucleophiles able to react with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides; they are involved in the addition of new C-C bonds to an starting carbon chain; therefore, this statement is a. True.
Best regards!
The energy change for an electronic transition in a one-electron atom or ion (H, He+, Li2+, etc.) from n initial to n final is given by delta E = -(2.18 times 10-18 J)(Z2)(1/n2 final - 1/n2 initial), where Z is the atomic number. Which one o f the following species will have the longest wavelength emission line for the transition between the n initial = 2 and n final = 1 levels? O H O He+ O Li2+ O Be3+
O B4+
Answers
H will have the longest wavelength emission line for the transition between the n initial = 2 and n final = 1 levels.
A movement (or leap) of an electron from one energy level to another within an atom or artificial atom is referred to as an atomic electron transition. It seems discontinuous because the electron "jumps" from one quantized energy level to another in a matter of nanoseconds or less.
Electronic transitions occur in atoms and molecules as a result of electromagnetic radiation absorption or emission (typically UV or visible). Planck's equation, E = h, relates the energy change associated with a transition to the frequency of an electromagnetic wave. Transitions between these permitted orbits occur in photon absorption or emission. A photon is released when an electron transitions from a higher-energy orbit to a more stable orbit.
To learn more about electronic transitions, here
https://brainly.com/question/18156550
#SPJ4
What evidence can be used to explain the type of mutualistic relationship present between the water buffalo and frog?
Answers
..................
are called energy giving nutrients
Answers
Answer:
macronutrients (carbohydrates, lipids, and proteins)
Robert Delaunay's Homage to Blériot (1914) was inspired by
O the invention of stroboscopic photography
the construction of the Eiffel Tower
his wife's new dress designs
the first flight across the English channel
Answers
Answer:
Robert Delaunay's Homage to Blériot (1914) was inspired by the first flight across the English channel.
Draw out a Simple electro Chemical Symbols in ions
Answers
Answer:
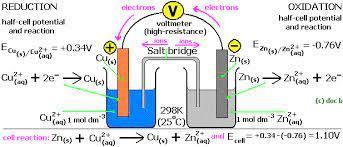
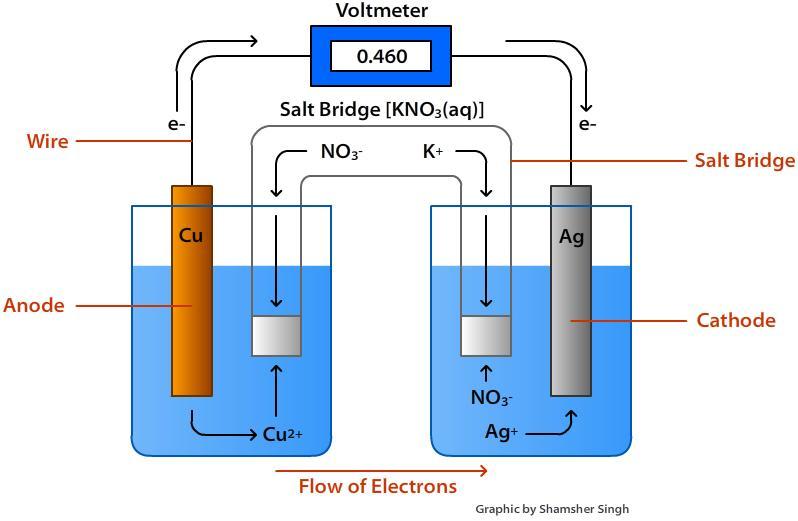
In redox reactions, electrons are transferred from one species to another. If the reaction is spontaneous, energy is released, which can then be used to do useful work. To harness this energy, the reaction must be split into two separate half reactions: the oxidation and reduction reactions. The reactions are put into two different containers and a wire is used to drive the electrons from one side to the other. In doing so, a Voltaic/ Galvanic Cell is created.
Explanation:
When a redox reaction takes place, electrons are transferred from one species to the other. If the reaction is spontaneous, energy is released, which can be used to do work. Consider the reaction of a solid copper (Cu(s)) in a silver nitrate solution (AgNO3(s)).
he AgNO3(s) dissociates in water to produce Ag+(aq) ions and NO−3(aq) ions. The NO3-(aq) ions can be ignored since they are spectator ions and do not participate in the reaction. In this reaction, a copper electrode is placed into a solution containing silver ions. The Ag+(aq) will readily oxidize Cu(s) resulting in Cu2+(aq), while reducing itself to Ag(s).
This reaction releases energy. When the copper electrode solid is placed directly into a silver nitrate solution, however, the energy is lost as heat and cannot be used to do work. In order to harness this energy and use it do useful work, we must split the reaction into two separate half reactions; The oxidation and reduction reactions. A wire connects the two reactions and allows electrons to flow from one side to the other. In doing so, we have created a Voltaic/ Galvanic Cell.
How many grams are in 9.97 moles of Be(NO3)2?
Use two digits past the decimal for all values.
Answers
Answer:
1,869.97 grams of Be(NO3)2
Explanation:
Be(NO3)2 = Be N2 O6
Be=9.012182g/mole
N2=28.0134g/mole
O6=96g/mole
therefore Be(NO3)2 gives you 187.56g in one mole
so 9.97 moles means there is 9.97 times more
9.97mole Be(NO3)2 * 187.56g Be(NO3)2/1mole Be(NO3)2 = 1,869.97g of Be(NO3)2
9. Which statement best describes the nucleus of
an aluminum atom?
A) It has a charge of +13 and is surrounded by
a total of 10 electrons.
B) It has a charge of + 13 and is surrounded
by a total of 13 electrons.
C) It has a charge of -13 and is surrounded by
a total of 10 electrons.
SD) It has a charge of -13 and is surrounded by
a total of 13 electrons.
Answers
Answer:
Its B)
Explanation:
i got it right
Aluminium is 13th element, containing 13 electrons and 13 protons. Thus the Al atomic nucleus has a charge of +13 and it is surrounded by 13 electrons.
What is aluminium?Aluminium is 13th element in periodic table. It is placed in 13th groups and it is a metal. Aluminium is an electropositive element and it conducts so that it is considered as a metal.
The nucleus of every element is composed of neutrons and protons. Neutrons are neutral and protons are positively charged particles. Electrons are negatively charged particles revolving around the nucleus.
The number of protons in an atom is called its atomic number. Hence, the nucleus of a aluminium contains 13 protons thus having +13 charge and equal number of electrons revolving around. Hence, option B is correct.
To find more on aluminum, refer here:
https://brainly.com/question/25869623
#SPJ6
Please I need help thank you

Answers
Answer:
its sodium hydroxide
Explanation:
Reflect on the learning activities titled “Hypothesis”, “Variables and Hypothesis” and “Constructing a Hypothesis”. Describe some similarities and differences between a question that comes in response to an observation, and a scientific research question? Cite quotes from the readings to support your answer. Where do variables fit into this thinking? In other words, if you imagine a number line with observation questions at one end and scientific research questions at the other, what role do variables play anywhere along this continuum?
Answers
Hypothesis is a proposed explanation for an observable phenomenon. The term comes from the Greek word for "to suppose." Variables, on the other hand, are anything that can be changed or measured. Variables can be independent, dependent, or control variables. Learning activities titled “Hypothesis”, “Variables and Hypothesis” and “Constructing a Hypothesis” share similarities and differences with a question that comes in response to an observation and a scientific research question.
On the other hand, "A scientific research question is more specific and usually relates to a hypothesis. For example, if you hypothesize that birds are attracted to gardens that have bird feeders, your research question might be, 'Does the presence of bird feeders in a garden attract more birds?'" Variables can fit anywhere along this continuum. Variables are anything that can be changed or measured. If you imagine a number line with observation questions at one end and scientific research questions at the other, variables can be used to test hypotheses, support or refute a claim. Variables can be independent, dependent, or control variables. Independent variables are variables that can be manipulated. Dependent variables are variables that depend on the independent variable. Control variables are variables that remain constant throughout the experiment.In conclusion, learning activities titled “Hypothesis”, “Variables and Hypothesis” and “Constructing a Hypothesis” share similarities and differences with a question that comes in response to an observation and a scientific research question. A question that comes in response to an observation is usually general and qualitative while a scientific research question is specific and quantitative. Variables can fit anywhere along the continuum and can be used to test hypotheses, support or refute a claim.For such more question on Hypothesis
https://brainly.com/question/606806
#SPJ8
If you have 1 liter of a 1 M solution of NaCl, how many moles of NaCl were dissolved in the water
to make that solution?
Answers
The number of moles of NaCl dissolved in the water to make the given solution is 1 mole.
What is a mole?A mole can be described as a unit that is employed to calculate the count of particles. The particles which are counted are usually identical entities, individually distinct.
The amount of chemical substance can be measured in the terms of the mole and the number of entities in one mole is approximately equal to 6.023 × 10 ²³.
Given, the one liter of a 1 M solution of NaCl.
The concentration of the NaCl solution = 1 M
The molarity of the given solution, \(M = n/V\)
The number of moles of NaCl, n = M ×V = \(\displaystyle {1 mol L^{-1}}\times{1L}\) = 1 mol
Learn more about the mole, here:
brainly.com/question/26416088
#SPJ1
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
36.8 g of CuSO4(s) was added to water to prepare a 2.00 L solution. What is the
concentration of CuSO4 in molarity?Molar mass of CuSO4 = 159.62 g/mol
A. 18.4 M
B. 1.84 M
C. 0.231 M
D. 0.115
Answers
Answer:
Option D. 0.115 M
Explanation:
The following data were obtained from the question:
Mass of CuSO4 = 36.8 g
Volume of solution = 2 L
Molar mass of CuSO4 = 159.62 g/mol
Molarity of CuSO4 =..?
Next, we shall determine the number of mole in 36.8 g of CuSO4.
This can be obtained as shown below:
Mass of CuSO4 = 36.8 g
Molar mass of CuSO4 = 159.62 g/mol
Mole of CuSO4 =.?
Mole = mass /Molar mass
Mole of CuSO4 = 36.8 / 159.62
Mole of CuSO4 = 0.23 mole
Finally, we shall determine the molarity of the CuSO4 solution as illustrated below:
Mole of CuSO4 = 0.23 mole
Volume of solution = 2 L
Molarity of CuSO4 =..?
Molarity = mole /Volume
Molarity of CuSO4 = 0.23 / 2
Molarity of CuSO4 = 0.115 M
Therefore, the molarity of the CuSO4 solution is 0.115 M.
Be sure to answer all parts.
A small hole in the wing of a space shuttle requires a 16.1 cm² patch.
(a) What is the patch's area in square kilometers (km²)? Enter your answer in scientific notation.
x 10
km²
(b) If the patching material costs NASA $2.94/in², what is the cost of the patch to the nearest cent?
Answers
A. The patch's area in square kilometers (km²) is 1.61×10⁻⁹ km²
B. The cost of the patch to the nearest cent is 734 cents
A. How to convert 16.1 cm² to square kilometers (km²)We can convert 16.1 cm² to km² as illustrated below:
Conversion scale
1 cm² = 1×10⁻¹⁰ km²
Therefore,
16.1 cm² = 16.1 × 1×10⁻¹⁰
16.1 cm² = 1.61×10⁻⁹ km²
Thus, 16.1 cm² is equivalent to 1.61×10⁻⁹ km²
B. How to determine the cost in centWe'll begin by converting 16.1 cm² to in². This can be obtained as illustrated below:
1 cm² = 0.155 in²
Therefore,
16.1 cm² = 16.1 × 0.155
16.1 cm² = 2.4955 in²
Finally, we shall the determine the cost in centas fo r llow:
Cost per in² = $2.94 = 294 centCost of 2.4955 in² =?1 in² = 294 cent
Therefore,
2.4955 in² = 2.4955 × 294
2.4955 in² = 734 cents
Thus, the cost of the patch is 734 cents
Learn more about conversion:
https://brainly.com/question/2139943
#SPJ1
Which of the following could be classified as unleavened bread?
sourdough bread
muffins
tortilla
bagels
Answers
Answer:
Tortilla
Explanation:
Answer:
tortilla
Explanation:
HELP ASAP!
The elements from which of the following groups are most likely to react with
magnesium(Mg)?
Group 18
Group 2
Group 16
Group 1
Answers
Answer:
oxygen
Explanation:
The elements from group 16 are most likely to react with magnesium (Mg). Therefore, option (3) is correct.
What are alkaline earth metals?Alkaline earth metals are the group 2 elements in the modern periodic table. They include six elements which are Beryllium(Be), Magnesium(Mg), Calcium(Ca), Strontium(Sr), Barium(Ba), and Radium(Ra).
Group 2 elements contain smooth, less metallic character than elements of group 1. The general valence shell electronic configuration of alkaline earth metals is ns².
All elements of this group have two valence electrons, giving them a valency of 2. These metals readily lose two electrons to form compounds through ionic bonds.
The valency of Magnesium is 2 and the valency of the elements of group 16 such as oxygen is also 2. They can combine in 1 : 1 ratio to form a neutral compound.
Mg²⁺ + O²⁻ → MgO
Therefore, the Magnesium will react with elements of group 16.
Learn more about alkaline earth metals, here:
brainly.com/question/11857448
#SPJ2
The equation below represents a chemical reaction at 1 atm and 298 K. N2(g) + 3H2(g) -+ 2NH3(g) State the change in energy that occurs in order to break the bonds in the hydrogen molecules
Answers
To break the bonds in the hydrogen molecules in the reaction N2(g) + 3H2(g) → 2NH3(g), approximately 1305 kJ of energy needs to be supplied.
In order to break the bonds in the hydrogen molecules (H2), energy needs to be supplied to overcome the attractive forces between the atoms within the molecules. Breaking bonds requires an input of energy and is an endothermic process.
In the given chemical reaction, N2(g) + 3H2(g) → 2NH3(g), the hydrogen molecules (H2) are broken as the reactants, N2 and H2, are converted into ammonia (NH3).
Breaking one H2 molecule requires the energy equivalent to the bond dissociation energy (also known as bond energy) of the H-H bond. The bond dissociation energy is the energy required to break one mole of a particular bond in a gaseous molecule.
The bond dissociation energy for the H-H bond is approximately 435 kJ/mol. This means that it takes approximately 435 kJ of energy to break one mole of H-H bonds.
In the given reaction, three moles of H2 molecules are involved. Therefore, the total energy required to break the bonds in the hydrogen molecules is:
Energy required = 3 moles * 435 kJ/mol = 1305 kJ
So, to break the bonds in the hydrogen molecules in the reaction N2(g) + 3H2(g) → 2NH3(g), approximately 1305 kJ of energy needs to be supplied.
It's important to note that breaking bonds requires energy input, while forming bonds releases energy. In this reaction, the formation of new bonds in the ammonia (NH3) molecules will release energy, resulting in an overall exothermic reaction. The energy change of the reaction, often referred to as the enthalpy change (ΔH), will depend on the difference between the energy required to break the bonds and the energy released when new bonds are formed.
for more such question on hydrogen visit
https://brainly.com/question/24433860
#SPJ8
‼️‼️‼️need help asap‼️‼️‼️

Answers
24. To calculate the molarity of a solution, we must first find out how many moles of \(BaI_2\) are in the solution.
Molar mass of BaI2 = (1 x atomic mass of Ba) + (2 x atomic mass of I)
= (1 x 137.33 g/mol) + (2 x 126.90 g/mol)
= 137.33 g/mol + 253.80 g/mol
= 391.13 g/mol
Number of moles of BaI2 = mass of BaI2 / molar mass of BaI2
= 413 g / 391.13 g/mol
= 1.056 mol
the molarity of the solution using the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
Volume of solution = 750 ml = 750 ml / 1000 ml/L = 0.750 L
Molarity = 1.056 mol / 0.750 L
= 1.408 M
Therefore, the molarity of the solution is 1.408 M.
25. a. \(P_20_7\) - Ionic compound (Phosphorus(V) oxide)
b. \(SnBr_2\) - Ionic compound (Tin(II) bromide)
c. \(Fe(OH)_2\)- Ionic compound (Iron(II) hydroxide)
d. \(Cl_30_8\) - Not a valid chemical formula
26.
A. (NH4)2CO3 is soluble in water (NH4) in an ionic substance called 2CO3 containing the ions carbonate and ammonium.
B. Fe(OH)2 is insoluble in water. Iron(II) hydroxide is only sparingly soluble.
C. CaOH is not soluble in water. Only very little calcium hydroxide is soluble.
D. PbCl2 is insoluble in water. The chloride of lead(II) is sparingly soluble.
27. FeS + 2KCl = FeCl2 + K2S
FeS is an insoluble precipitate.
2KCl dissolves in aqueous solution.
ZnCl2 + SrSO4 = ZnSO4 + SrCl2
SrSO4 is an insoluble precipitate.
ZnCl2 dissolves in aqueous solution.
28. In salt water, the solute is the salt (sodium chloride, or NaCl), and the solvent is water. The element which dissolves in the solvent to form a solution is called solute.
29. Charles's law states that, if the pressure and volume of a gas remain constant, the volume of a gas falls as the temperature increases. As a result, the capacity of the balloon will decrease as it ascends to altitudes where the temperature is -15 °C.
30. The average kinetic energy of the particles of a substance increases with increase in its temperature. This is because temperature is a gauge for the specific kinetic energy of the constituent particles of a substance. On the other hand, the average kinetic energy falls as the temperature increases.
31. When the volume of a gas decreases, its pressure increases. Boyle's law, which states that at a given temperature, the pressure of a gas is inversely proportional to its volume, describes this relationship. On the other hand, pressure falls when volume increases.
32. The pressure of a gas increases along with its temperature. Gay–Lussac's law, which states that the pressure of a gas is directly proportional to its temperature, given the volume and volume of the gas is constant, describes this relationship.
33. The volume of a syringe is reduced as a marshmallow is pressed and the plunger is depressed. As a result the pressure inside the syringe increases. This is because Boyle's law states that the volume and pressure of a gas are inversely proportional. The decrease in volume causes the air inside the syringe to contract, exerting more pressure on the marshmallow, which is then crushed.
Learn more about Charles's law, here:
https://brainly.com/question/12835309
#SPJ1
calculate the hydrogen ion concentration of a solution who's pH is 2.4
Answers
Answer:
I don't know sorry yyyyyyy6yyyyyyyyyyyyyyyyyyyyyyyyyyy
////////////////////////////////////////////////////////
\
Answers
Answer:
///////////////////////////////////\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\/\
Explanation:
Write a formula for the ionic compound that forms from magnesium
and oxygen.
Answers
Answer:
MgO
Explanation:
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.