Answers
Answer:
Bromine
Explanation:
Cuz it has bro in it :)
Related Questions
What are the properties of gas
Answers
Answer:
1) easy compressed
2) fills its container
3) far more space
Explanation:
Indefinite Shape or Volume. Gases have no definite shape or volume. ...
Compressibility and Expandability. ...
Diffusivity. ...
Pressure.
5.00 mol of ammonia are introduced into a 5.00 L reactor vessel in which it partially dissociates at high temperatures. 2NH 3(g) 3H 2(g) + N 2(g) At equilibrium and a particular temperature, 1.00 mole of ammonia remains. Calculate K c for the reaction.
Answers
Explanation:
system at equilibrium, will the reaction shift towards reactants ~
--?'
2. (2 Pts) Consider the reaction N2(g) + 3H2(g) =; 2NH3(g). The production of ammonia is an
exothermic reaction. Will heating the equilibrium system increase o~e amount of
ammonia produced? . .co:(
3. (2 Pts) Consider the reaction N2(g) + 3H2(g) =; 2NH3(g). Ifwe use a catalyst, which way will
the reaction shift? ':'\
.1.+- w~t s~,H (o')l r'eo.c. e~ ei~i"liht-,·u.fn\ P~~,
4. (3 Pts) ff 1ven th e o £ 11 owmg d t a a £ or th ere action: A(g) + 2B(s) =; AB2(g)
Temperature (K) Kc
300 1.5x104
600 55 k ' pr, cl l<..J~
e- ~ r fee, ct o. ~ 1<
900 3.4 X 10-3
Is the reaction endothermic or exothermic (explain your answer)?
t d- IS o.,;r-. \4\a..i~1f't~ °the te.Y'il(lf1,:J'u.r-a a•~S. j lrvdu..c,,.) +~H~to{' '\
exothe-rnh't.-- ,.. ..,. (/.., ,~.
5. (4 Pts) Consider the reaction, N2(g) + 3H2(g) =; 2NH3(g). Kc= 4.2 at 600 K.
What is the value of Kc for 4 NH3(g) =; 2N2(g) + 6H2(g)
N ... ~l + 3 H~(ri ~ ~Nli3~) kl,= ~:s;H,J3 # 4. J..
~ ;)N~~) ~ ~ H ~) ~\-_ == [A!;J:t D~~Jb
J. [,v 1+3] ~
I
4,:i.~ = 0,05
5.00 mol of ammonia are introduced into a 5.00 L reactor vessel and when the equilibrium is reached, 1.00 mole remains. The concentration equilibrium constant is 17.3.
Initially, there are 5.00 mol of ammonia in a 5.00 L reactor vessel. The initial concentration of ammonia is:
\([NH_3]_i = \frac{5.00mol}{5.00L} = 1.00 M\)
At equilibrium, there is 1.00 mole of ammonia in the 5.00 L vessel. The concentration of ammonia at equilibrium is:
\([NH_3]eq = \frac{1.00mol}{5.00L} = 0.200 M\)
We can calculate the concentrations of all the species at equilibrium using an ICE chart.
2 NH₃(g) ⇄ 3 H₂(g) + N₂(g)
I 1.00 0 0
C -2x +3x +x
E 1.00-2x 3x x
Since the concentration of ammonia at equilibrium is 0.200 M,
\(1.00-2x = 0.200\\\\x = 0.400 M\)
The concentrations of all the species at equilibrium are:
\([NH_3] = 0.200 M\\[H_2] = 3x = 1.20 M\\[N_2] = x = 0.400 M\)
The concentration equilibrium constant (Kc) is:
\(Kc = \frac{[H_2]^{2} [N_2]}{[NH_3]^{2} } = \frac{(1.20^{3})(0.400) }{0.200^{2} } = 17.3\)
5.00 mol of ammonia are introduced into a 5.00 L reactor vessel and when the equilibrium is reached, 1.00 mole remains. The concentration equilibrium constant is 17.3.
Learn more: https://brainly.com/question/15118952
PLEASE john travoltage is safe from getting a static electricity zap right now. there are two things that could change to increase his chances from getting zapped tell me what both if those things are

Answers
If he rubs his foot on the carpet or touches the metal doorknob it would increase his chances of being zapped.
Rubbing his feet would be friction.
Touching the doorknob would be potential energy.
Side note: I love the format for this question, was not expecting to see John Travolatge on here today >O< !!!
Daniella found an iron metal cube. The volume of the cube is 20 cm3 as shown below.
non
20.0 cm
If the density of iron is 7.9 g/cm3, what is the mass in grams of this iron cube?
A) 0.395 g
B) 2.53 g
C) 27.99
D) 158 g
Answers
Answer:b) 2.53
Explanation:
What is the number of molecules in
500m cube of Oxygen at room temperature
Answers
4.95 is the number of molecules in 500m cube of Oxygen at room temperature and pressure
we have got data
pressure=1atm
temperature=298 k
volume=500 m^3
gas constant, R=8.31
now, by applying ideal gas equation
PV=nRT
1×500=n×8.31×298
n=2476.38/500
n=4.95
It is the lowest part of the material and has chemical elemental properties. Atoms do not independently exist; rather, they combine to form ions and molecules, which then combine to form the material that we can see, feel, and touch.
Molecules are made up of one or more atoms connected by covalent (chemical) connections. Atoms can be visualized as circles with a nucleus in the center (made up of protons and neutrons) and one or more concentric circles around it that indicate the "shells" or "levels" in which the electrons surrounding the atom's nucleus are located, as well as markings that identify the electron. each level
To know more about molecules visit : https://brainly.com/question/11405437
#SPJ9
hydrogen and oxygen reacts chemically to form water. how much water would form if 14.8 grams of hydrogen reacted with 34.8 grams of oxygen
Answers
The mass of water that can be obtained is 2.2 g of water.
What is the mass of the water?We know that in the case that we have here, we are going to depend on the stoichiometry of the reaction and this is very important in the determination of the amount of the water that can be formed.
Now we can see that the reaction equation can be written from the fact that; \(2H_{2} (g) + O_{2} (g) ---- > 2H_{2} O(g)\). Then we can see that in this kind of chemical reaction we have to apply the stoichiometry.
Number of moles of the hydrogen = 14.8g/2 g/mol = 7.4 moles
Number of moles of oxygen = 34.8 g/32 g/mol = 1.1 moles
Given that 2 moles of hydrogen reacts with 1 mole of oxygen
7.4 moles of hydrogen reacts with 7.4 * 1/2
= 3.7 moles
Hence oxygen is the limiting reactant.
1 mole of oxygen produces 2 moles of water
1.1 moles of oxygen would produce 1.1 * 2/1
= 2.2 moles of water
Learn more about limiting reactant:https://brainly.com/question/14225536
#SPJ1
What is intermolecular force between bromine and benzene?
Answers
The intermolecular force between bromine and benzene is primarily a van der Waals force known as London dispersion forces.
London dispersion forces occur due to temporary fluctuations in electron distribution, creating temporary dipoles in molecules. In the case of bromine and benzene, both molecules are nonpolar, meaning they have no permanent dipole. However, they still experience London dispersion forces.
Benzene is a cyclic aromatic hydrocarbon with a hexagonal ring structure. It consists of delocalized π electrons above and below the plane of the molecule. Bromine is a halogen element with seven valence electrons, which forms diatomic molecules. In the solid or liquid state, bromine molecules exist as Br2.
The London dispersion forces between bromine and benzene arise from the temporary shifts in electron density within their electron clouds. The π electrons in benzene induce temporary dipoles in the bromine molecules, resulting in attractive forces between them. These temporary dipoles continuously form and break due to electron movements, resulting in an overall attractive force between the bromine and benzene molecules.
While London dispersion forces are generally weaker than other intermolecular forces like dipole-dipole interactions or hydrogen bonding, they still contribute to the stability of the system and affect properties such as boiling points and melting points.
For such more questions on intermolecular
https://brainly.com/question/26497701
#SPJ8
a sample of gas occupies a volume of 2.62 liters at 25 C and 1.00 atm. what will be the volume at 50 C and 2 atm
Answers
Answer:2.62 L
Explanation:
A sample of gas occupies a volume of 2.62 liters at 25° C and 1.00 atm. and the volume at 50° C and 2 atm then volume is 2.62 liters.
What is ideal gas law ?The equation of state for a fictitious perfect gas is known as the ideal gas law, sometimes known as the generic gas equation. Although it has significant drawbacks, it is a decent approximation of the behavior of many gases under various situations.
An ideal gas is one in which there are no intermolecular attraction forces and all collisions between atoms or molecules are entirely elastic. It may be seen as a group of perfectly hard spheres that collide but do not else interact with one another.
By using ideal gas equation,
P₁ V ₁ ÷ T = P₂V₂ ÷ T
1 × 2.62 ÷ 25 = 2 × V₂ ÷ 50
V₂ = 1 × 2.62 × 50 ÷ 25 × 2
V₂ = 2.62 liters.
Thus, a sample of gas occupies a volume of 2.62 liters at 25° C and 1.00 atm. and the volume at 50° C and 2 atm then volume is 2.62 liters.
To learn more about ideal gas law follow the link below;
https://brainly.com/question/6534096
#SPJ5
Identify Cause and Effect
Directions: Read the pairs of statements below. On the line next to each statement,
write C if the statement is a cause and E if the statement is an effect. The first one is
done for you.
C
E
1. Wood is burned in a fireplace.
Thermal energy is released.
2. Carbon dioxide is formed.
Carbon combines with oxygen.
3. Solar panels can collect solar energy.
Solar energy is given off by the sun.
4. Thermal energy is released when coal is burned.
Answers
1. C (Cause: Wood is burned in a fireplace; Effect: Thermal energy is released.)
2. E (Cause: Carbon combines with oxygen; Effect: Carbon dioxide is formed.)
3. C (Cause: Solar panels can collect solar energy; Effect: Solar energy is given off by the sun.)
4. C (Cause: Thermal energy is released when coal is burned; Effect: Thermal energy is released)
Write down a balanced equation for SnO2 + H2 → Sn + H2O and tell which substance is the oxidising agent and which is the reducing agent.
Answers
Answer:
Sn is the oxidation agent and h2 is the reducing agent
Explanation:
Because oxidation agent means reduction which means the lose of oxygen and Sn lose oxygen.
While reduction agent means oxidation which also means the gain of oxygen and h2 gain oxygen.
I hope you understand my explanation if you need any help in chemistry I'm always here
what's the importance of anatomy for pharmacy students
Answers
All health professionals, including pharmacists, must be able to relate form to function..a grounding in anatmony is an essential foundation on which to underpin other knowledge relevant to clinical practice.
A solution contains 1.0 x 10-5 M Na3PO4. What is the minimum concentration of AgNO3 that would cause precipitation of solid Ag3PO4 (ksp = 1.8 x 10-18)?
Answers
The minimum concentration of AgPO4 that will cause precipitation of Ag3PO4 is \(5.64*10^-^5M\)
Data;
Ksp of Ag3PO4 = 1.8*10^-18concentration of Na3PO4 = 1.0*10^-5 MThe KSP value of the Precipitate\(ksp = [Ag]^3[PO_4]\)
The ksp value of \(AgPO_4 = 1.8*10^-^1^8\\\)
Let's substitute the values and solve for the cation
\(1.8*10^-^1^8= [Ag]^3[1.0*10^-^5]\\\\\)
This becomes
\([Ag]^3=\frac{1.8*10^-^1^8}{1.0*10^-^5}\\ \\\)
\([Ag]=\sqrt[3]{1.8*10^-^1^3}\\\\\)
\([Ag] = 5.64 * 10 ^-5M\)
From the above calculation, we can say that the minimum concentration of AgPO4 that will cause precipitation of Ag3PO4 is 5.64*10^-5M
Learn more on solubility and ksp value of solutions here;
https://brainly.com/question/15546566
https://brainly.com/question/9866138
please help i don’t know this:(

Answers
Answer:
29.5 days
Explanation:
orginally 27.3 days but 29.5 days is also correct
Copper reacts with 36.7 g of silver nitrate to produce copper(II) nitrate and silver. Determine the theoretical yield of Cu(NO3)2 (show work)
Answers
The theoretical yield of Cu(NO3)2 is 20.259 grams.
To determine the theoretical yield of Cu(NO3)2, we need to calculate the amount of copper reacting with the silver nitrate and use stoichiometry to convert that amount to the molar mass of Cu(NO3)2.
First, let's find the molar mass of silver nitrate (AgNO3):
AgNO3 = 107.87 g/mol (Ag) + 14.01 g/mol (N) + 3 * 16.00 g/mol (O) = 169.87 g/mol
Next, we need to calculate the amount of copper that reacts with the given mass of silver nitrate. The molar mass of copper (Cu) is 63.55 g/mol.
Using the molar ratio from the balanced chemical equation, we can relate the moles of copper to the moles of silver nitrate. The balanced equation is:
Cu + 2AgNO3 -> Cu(NO3)2 + 2Ag According to the equation, 1 mole of copper reacts with 2 moles of silver nitrate.
Now we can calculate the moles of copper reacting with 36.7 g of silver nitrate:
moles of AgNO3 = (mass of AgNO3) / (molar mass of AgNO3)
moles of AgNO3 = 36.7 g / 169.87 g/mol = 0.2160 mol AgNO3
Using the stoichiometric ratio, we find the moles of Cu:
moles of Cu = (moles of AgNO3) / (2 moles of AgNO3 per 1 mole of Cu)
moles of Cu = 0.2160 mol AgNO3 / (2 mol AgNO3/1 mol Cu) = 0.1080 mol Cu
Finally, we can calculate the theoretical yield of Cu(NO3)2 using the molar mass of Cu(NO3)2:
theoretical yield of Cu(NO3)2 = (moles of Cu) * (molar mass of Cu(NO3)2)
theoretical yield of Cu(NO3)2 = 0.1080 mol * (63.55 g/mol + 2 * (14.01 g/mol + 3 * 16.00 g/mol))
theoretical yield of Cu(NO3)2 = 0.1080 mol * 187.56 g/mol
theoretical yield of Cu(NO3)2 = 20.259 g
For more such questions on theoretical yield visit:
https://brainly.com/question/14714924
#SPJ11
what looks attractive on guys
Answers
Answer: for me everything ig
Explanation:
Describe how to prepare 400 grams of a 15% (mass/mass) aqueous solution of KBr.
Answers
Dissolve 60g of potassium bromide in 340g of water to produce 15% (mass/mass) aqueous solution of potassium bromide.
Here we have to prepare a total of 400 g of solution. Aqueous solution means the solvent we use here is water.
So to prepare 400 g of 15% aqueous solution of potassium bromide, we need to find out how many grams of potassium bromide need to be dissolved in water and how many grams of water must be used.
Here the weight percent is given, that is 15%
15/100 = weight of potassium bromide/ 400 g
0 .15 = weight of potassium bromide / 400
weight of potassium bromide needed = 0.15 × 400
= 60 g
So, we calculated the required amount of potassium bromide as 60 grams. The total weight of the solution to be made is 400 grams.
So amount of water required = 400 - 60
= 340 g
So we need to mix 60 grams of potassium bromide in 340 grams of water to get a 15% (mass/mass) aqueous solution.
For further information about preparing aqueous solutions, please refer
https://brainly.com/question/13684060
PLEASE ANSWER QUICKLY!!
100 NaNO3
90
Solute per 100 g of H₂O (g)
0,80
NH,CI
70 KNO3
60
50
40
30
20
10
0
0 10 20 30 40 50 60 70 80 90 100
Temperature (°C)
KCIO3
60 g KNO3 has
been added to
100 g H₂O at
30 °C. What
type of solution
is this?
A. unsaturated
B. saturated
C. supersaturated

Answers
If 60 grams of the substance are added to 100 g of water, the solution can be categorized as supersaturated.
How saturated is this solution?The graph shows the number of grams that can be dissolved in 100 grams of water at different temperatures. In general, solubility increases with temperature.
According to the graph, at a temperature of 30°C, it is possible to dissolve a total of 48 to 49 grams of \(KNO_{3}\). This information implies that if we add 60 grams at this temperature not all the substance would be dissolved, and therefore the solution would be supersaturated.
Learn more about solubility in https://brainly.com/question/31493083
#SPJ1
In three to five sentences, identify the type of bonding that would form between potassium (K) and chlorine (Cl). Use electronegativity to justify this bond type. Provide the chemical formula for the resulting compound.
(two sentences is good enough tbh)
Answers
Answer:
when potassium reacts with chlorine, the former loses its valence electron and the latter takes it. The two resulting ions, i.e. the potassium cation and the chloride anion, are then bonded together by the electrostatic force of attraction → an ionic bond is formed.
Explanation:
One ion of the element Potassium combine with one ion of the element chlorine to form the compound potassium chloride in fixed proportion
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
There are two types of compound, covalent compound and ionic compound. Covalent compound are formed by sharing of electron and ionic compound formed by complete transfer of electron.
According to our question sodium is a metal so it can donate its electron to chlorine and hence form ionic bond. Chlorine is more electronegative than sodium so it can take electron from sodium easily.
The balanced equation is
Na⁺+Cl⁻\(\rightarrow\) NaCl
So from this we can see that One atoms of element Potassium combine with one atom of the element chlorine to form the ionic Compound Potassium chloride.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ5
Please help I will give brainiest‼️‼️‼️‼️‼️‼️‼️
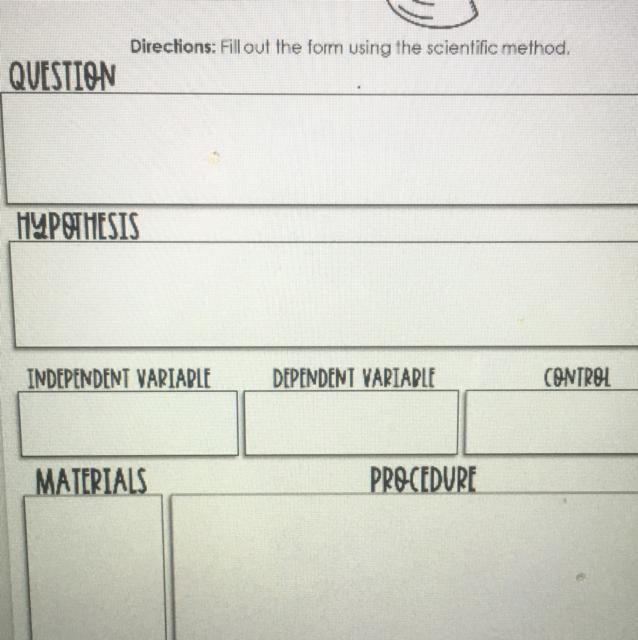
Answers
The scientific method is the method used for scientific discovery.
What is the scientific method?The scientific method is the process or procedure which scientists follow in their discovery and explanation of natural phenomena.
The scientific method is as follows:
Observations/QuestionsHypothesisExperimentsResultsConclusionsUsing the scientific method to determine if water is needed by plants to grow
Question: Is water needed for growth in plants?
Hypothesis: Plants need water
Independent variable: sunlight
Dependent variable: rate of evolution oxygen
Constant variable: sunlight and soil
Materials: Cupboard, soil, potted plant, water,
Procedure: One potted potted plant is watered, while the other is not watered. The length of the plant is measured after 10 days
Observation: The watered plant survived and grew while that which was not watered did not grow but died.
Conclusion: water is needed for growth in plants.
In conclusion, the scientific method can be used to determine if plants need water for growth.
Learn more about scientific method at: https://brainly.com/question/17274244
#SPJ1
A sample containing 16.93 metal pellets is poured into a graduated cylinder containing 11.2 of water, causing the water level in the to 19.7 Calculate the density of the metal.
Blank = g/ml
Answers
Answer:
The answer is 1.99 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 16.93 g
volume = final volume of water - initial volume of water
volume = 19.7 - 11.2 = 8.5 mL
We have
\(density = \frac{16.93}{8.5} \\ = 1.9917647058...\)
We have the final answer as
1.99 g/mLHope this helps you
in Newton's second law what forces cause an object to accelerate
Answers
In Newton's second law, unbalanced or net forces cause an object to accelerate.
Newton's second law of motion states that the acceleration of an object is directly proportional to the net force acting on it and inversely proportional to its mass. It can be expressed as F = m × a
Where:
F is the net force acting on the object,
m is the mass of the object,
a is the acceleration produced in the object.
This law describes the relationship between force, mass, and acceleration. The greater the force applied to an object, the greater its acceleration. The greater its mass, the smaller its acceleration for the same force.
Learn more about Newton's second law, here:
https://brainly.com/question/27712854
#SPJ1
Is plastic pollution likely to become more or less of a problem in the future?
Answers
Answer:
More bro
Explanation:
Pollution is only increasing
Answer: Yes and no, no because people are constantly polluting the earth with plastic which is a big issue. Yes, because things are being invented to help with plastic pollution, and there is a great number of people who recycle, so however you look at it there's really a 50/50 chance.
Explanation:
The atomic number of an atom is always equal to the total number of
A. neutrons in the nucleus
B. protons in the nucleus
C. neutrons plus protons in the atom
D. protons plus electrons in the atom
Answers
Answer:
yep C is correct!!
Explanation:
33. At the right of a chemical equation are
A. Reactants
B. Products
C. Coefficients
D. None of the above
Answers
Answer:
Reactant
Explanation:
hope this helps you
Assume you have 4 solids (A, B, C and D) of similar mass. Which of these requires the greatest energy input to melt?
polar covalent
covalent network
ionic compound
nonpolar covalent
Answers
The solid that require the greatest energy input to melt by mass is the option;
Covalent network
Reason for the above answer is as follows;
The elementary particles of a solid are held together by bonds that require
an input of energy to unlock, and once broken, the particles are then able
to change location within their containing vessels with less restrictions
Types of bonds
Polar covalent molecular solids have the following characteristics;a) Soluble in water b) Low melting point, b) Conduct electricity
Solids that are made up of a covalent network have the following characteristicsa) High melting point temperature b) Non conductive of electricity c) Not soluble in water
Solids of ionic compounds have the following characteristics;a) High melting point temperature b) The liquid state and solution
conducts electricity c) Soluble in water
Solids that have nonpolar covalent bonds have;a) Low melting point b) Normally in the gaseous or liquid state b) Not water soluble
Therefore, the covalent network, and the solids ionic compounds require the most energy to melt, however, the strength of the ionic bond in an ionic compound is a factor the charges present and the sizes of the atom, while
the covalent network solid, are combined to form essentially as a single
molecule and therefore require the greatest heat energy input break the bonds of the molecule down in order to melt
Learn more about the properties of the different types of bonds here;
https://brainly.com/question/13510199
https://brainly.com/question/21628972
https://brainly.com/question/14823280
HELP HELP HELP
how are scientist using computer models to simulate worldwide ocean activity
Answers
Answer:
An ocean current is a continuous, directed movement of seawater generated by forces acting upon this mean flow, such as breaking waves, wind, the Coriolis effect, cabbeling, and temperature and salinity differences. Features like depth contours, shoreline configurations, and interactions with other currents also influence an ocean current’s direction and strength. Ocean currents flow for great distances, and together, create the global conveyor belt that plays a dominant role in determining the climate of many of the Earth’s regions (illustrated in the NASA video below). For example, the Gulf Stream makes northwest Europe much more temperate than any other region at the same latitude. Another example is Lima, Peru where the climate is cooler than the tropical latitudes in which the area is located, due to the effect of the Humboldt Current. Ocean modelling uses a mathematical model of the general circulation of an ocean, based on the Navier–Stokes equations on a rotating sphere with thermodynamic terms for various energy sources (radiation, latent heat). These equations are the basis for complex computer programs commonly used for simulating the atmosphere or ocean of the Earth.
Explanation:
A 10.0-mL sample of an unknown H3PO4 solution requires 112 mL of 0.100 M KOH to completely react with the H3PO4. What was the concentration of the unknown H3PO4 solution
Answers
Answer:
The correct answer is 0.373 M.
Explanation:
The reaction will be,
H3PO4 (aq) + 3KOH (aq) ⇔ 3H2O (l) + K3PO4 (aq)
Based on the given information, the molarity of the solution KOH is 0.100 M, the volume of the solution given is 112 ml or 0.112 L.
The molarity of the solution with respect to KOH is,
M = Moles/Volume of Solution (liters)
Moles of solute = Molarity * Volume of solution
Moles = 0.100 M * 0.112 L
= 0.0112 moles
From the reaction it is clear that 3 moles of KOH needed 1 mole of H3PO4, thus, 0.0112 moles of KOH will require,
1/3 * 0.0112 = 0.00373 moles of H3PO4
The volume of the H3PO4 solution is 10 ml or 0.01 L.
The molarity of the solution will be,
M = Moles/ Volume of Solution (liters)
M = 0.00373/0.01 = 0.373 M
Thus, the concentration of the solution is 0.373 M.
The concentration of the H3PO4 would be 0.37 M
Going by the balanced equation of the reaction:
\(3KOH + H_3PO_4 ---> 3H_2O + K_3PO_4\)
The mole ratio of KOH to H3PO4 is 3:1.
Using the equation: CaVa/CbVb = 1/3
What we are looking for is Ca:
Ca = CbVb/3Va
= 0.1 x 112/3 x 10
= 0.37 M
More or calculations involving neutralization reactions can be found here: https://brainly.com/question/7305038?referrer=searchResults
Need help asap
1. All nonmetals (except hydrogen) have 8 valence electrons?
True or False
2. The N^-3 ion is classified as a(n) ____ and has ____.
A. anion, 8 valence electrons
B. cation, 8 valence electrons
C. anion, 15 valence electrons
D. anion, 3 valence electrons
3. If two nonmetals react to form a compound and have very different _____ they form ____ bonds. If there is a small difference, then they form ____ bonds.
A. ionization energy; covalent; nonpolar ionic
B. electronegativity; nonpolar covalent; polar covalent.
C. ionization energy, ionic, nonpolar covalent
D. electronegativity, polar covalent, nonpolar covalent.
Answers
Answer:
1: false
2: B
Explanation:
I do not know the 3rd one
a. Use the acid-base reaction HBr + LIOH → LiBr + H₂O to answer the following q
i. Name the Arrhenius acid and Arrhenius base found in the above reaction.
Answers
Answer:
Arrhenius Acid = HBr
Arrhenius Base = LiOH
Explanation:
Arrhenius acids are substances which increase the H⁺ concentration of an solution it has been dissolved in.
Arrhenius bases are substances which increase the OH⁻ concentration of a solution it has been dissolved in.
When dissolved in a solution, HBr dissociates into H⁺ and Br⁻ ions. As such, HBr increases the H⁺ concentration of the solution and is a Arrhenius acid.
When dissolved in a solution, LiOH dissociates into Li⁺ and OH⁻ ions. As such, LiOH increases the OH⁻ concentration of the solution and is an Arrhenius base.
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.