Answers
Answer:
B: Olfactory Bulb
Explanation:
edg2020
The olfactory bulb is located at the base of the brain and receives neural signals about the smell from the sensory receptors. Hence, option B is correct.
What are sensory receptors?Sensory receptors are dendrites of sensory neurons specialized for receiving specific kinds of stimuli.
There are two olfactory bulbs on the bottom side of the brain, one above each nasal cavity.
The olfactory bulbs receive information about smells from the nose and send it to the brain by way of the olfactory tracts.
Hence, option B is correct.
Learn more about sensory receptors here:
https://brainly.com/question/9911075
#SPJ2
Related Questions
The three states of matter differ primarily in terms of shape and volume. Describe solids, liquids and gases in these terms.
Answers
Answer:
solids have definite shape and volume. liquid have definite volume but not definite shape. gases do not have definite volume as well as definite shape.
PLEASE HELP ASAP! WILL MARK YOU AS BRAINLIEST!!!

Answers
Answer:
what are the options?
Explanation:
Which example is a biotic factor of an aquarium environment?
Responses
amount of oxygen in the water
water temperature
amount of sand in the aquarium
number of underwater plants
Answers
To solve this we must be knowing each and every concept related to environment. Therefore, the correct option is option B among all the given options.
What is environment?An environment may be simply defined as a system that includes all abiotic and biotic components that have an impact on human life. All flora and animals are considered biotic, or living, elements, whereas water, sunshine, air, temperature, etc. are considered abiotic.
Any good, service, or feature that benefits people and society might be considered one of an environment's resources. They might be anything that fulfills a person's requirements on a daily basis. Amount of oxygen in the water is a biotic factor of an aquarium environment.
Therefore, the correct option is option B.
To know more about environment, here:
https://brainly.com/question/28962722
#SPJ1
Answer: B: Water Temperature
Explanation: k12 test 1.04 Science Life semester 2
3. Joe is responsible for escorting some visiting clients around town. They are here for an important meeting, and his boss wants him to take them out for
a nice dinner and to a basketball game. However, their flight was delayed and the schedule Joe made is now several hours off. What skill will BEST help
him make the evening work?
OA. fluency
flexibility
OB flexibility
OC. computer skills
OD. critical thinking
Answers
Joe is responsible for escorting some visiting clients around town. They are here for an important meeting, and his boss wants him to take them out and their flight was delayed and the schedule Joe made is now several hours off therefore the skill which will best help him make the evening work is Flexibility and is therefore denoted as option B.
What is Flexibility?This is an important skill in a workplace and it involves an individual being able to quickly adapt to new circumstances as they arise.
In this scenario, the change in schedule means he will need this skill so as to be able to make the evening work through the making of different adjustments.
Read more about Flexibility here https://brainly.com/question/27356012
#SPJ1
A sample of 2.0 grams of helium gas is contained in a tank with a volume of 5.0 L at a temperature of 25° C. what is the pressure of the gas in the tank in atm?
Answers
PV = nRT
where P is the pressure of the gas, V is the volume of the tank, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature of the gas in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin by adding 273.15 to it:
T = 25°C + 273.15 = 298.15 K
Next, we need to calculate the number of moles of helium gas in the tank using its mass and molar mass:
n = m/M
where m is the mass of the gas and M is its molar mass. The molar mass of helium is approximately 4.00 g/mol.
n = 2.0 g / 4.00 g/mol = 0.50 mol
Now we can substitute the values we have into the ideal gas law equation and solve for P:
P = nRT/V
P = (0.50 mol)(0.08206 L·atm/(mol·K))(298.15 K)/(5.0 L)
P = 2.43 atm
Therefore, the pressure of the helium gas in the tank is approximately 2.43 atm.
Liquid nitrogen is a very cold fluid form of nitrogen that is capable of freezing objects in very short periods of time. In fact, if a ping pong ball is placed in liquid nitrogen for a few minutes and then dropped on the floor, it shatters like glass.
What type of changes does the ball experience when it is frozen in the liquid nitrogen and then dropped on the floor?
A.
physical changes only
B.
neither physical nor chemical changes
C.
physical and chemical changes
D.
chemical changes only
plz don't give wrong asnwer
Answers
Answer:
Answer C physical and chemical
How many moles are in 0.1 g of Magnesium?
Answers
Answer:
there are approximately 0.004118 moles in 0.1 g of magnesium.
Explanation:
The molar mass of magnesium is approximately 24.31 g/mol. To calculate the number of moles in 0.1 g of magnesium, we can use the following formula:
Number of moles = Mass / Molar mass
Number of moles = 0.1 g / 24.31 g/mol
Number of moles = 0.004118 mol (rounded to 3 significant figures)
Therefore, there are approximately 0.004118 moles in 0.1 g of magnesium.
Answer:
Explanation:
To calculate the number of moles of magnesium in 0.1 g of magnesium, we first need to determine the molar mass of magnesium. The molar mass of magnesium is 24.31 g/mol.
Using this information, we can use the following formula to calculate the number of moles of magnesium:
moles of magnesium = mass of magnesium / molar mass of magnesium
moles of magnesium = 0.1 g / 24.31 g/mol
moles of magnesium ≈ 0.00412 mol
Therefore, there are approximately 0.00412 moles of magnesium in 0.1 g of magnesium.
If it takes 0.0393 L of oxygen gas kept in a cylinder under pressure to fill an evacuated 1.05 L reaction vessel in which the pressure is 0.980 atm, what was the initial pressure of the gas in the cylinder
Answers
Answer:
26.2 atm
Explanation:
P1=P2V2/V1
P1=(1.05 x .980)/.0393
Answer: 26.2 atm
You can check this by knowing that P and V at constant T have an inverse relationship. Hence, this is correct.
Precipitation of an ionic compound will occur upon mixing of desired reagents if the initial ion product is:_______
A) greater than the Ksp
B) equal to the pksp
C) equal to the Ksp
D) less than the Ksp
Answers
Answer:
A) greater than the Ksp
Explanation:
Given a solid ionic compound AB, it dissociates in water into its ions, as follows:
AB(s) → A⁺(aq) + B⁻(aq)
At equilibrium, the product of the concentrations of the ions is constant, and it is called Ksp:
AB(s) ⇄ A⁺(aq) + B⁻(aq)
Ksp = [A⁺][B⁻] ⇒ (concentrations at equilibrium)
Upon mixing the reagents for the formation of AB, the compound will precipitate if the initial ion product (Q) is greater than the Ksp. If Q is equal to Ksp, the ions are at equilibrium with the solid compound AB, and if is it less than the Ksp, the ions are soluble and no solid AB is formed yet.
Q = [A⁺][B⁻] ⇒ (initial concentrations)
Q = Ksp ⇒ saturated solution (at equilibrium)
Q< Ksp ⇒ unsaturated solution (ions are soluble)
Q> Ksp ⇒ precipitation of solid compound.
Therefore, the correct option is A) greater than the Ksp
How many moles of MgCl2 are present in 60.0 mL of 0.100 M MgCl2 solution
Answers
Taking into account the definition of molarity, the number of moles of MgCl₂ present in 60.0 mL of 0.100 M MgCl₂ solution is 0.006 moles.
Definition of molarityMolarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by:
molarity= number of moles of solute÷ volume
Molarity is expressed in units moles/L.
Number of moles of MgCl₂In this case, you have:
Molarity= 0.100 Mnumber of moles of MgCl₂= ?volume= 60 mL= 0.06 L (being 1000 mL= 2 L)Replacing in the definition of molarity:
0.100 M=number of moles of MgCl₂÷ 0.06 L
Solving:
0.100 M × 0.06 L= number of moles of MgCl₂
0.006 moles= number of moles of MgCl₂
Finally, the number of moles of MgCl₂ is 0.006 moles.
Learn more about molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
#SPJ1
Why would this path of neurons not work to perform a reflex?
Select all that apply.
the motor neuron should be the interneuron
the motor neuron should be the sensory neuron
the sensory neuron should be the interneuron
the interneuron should be the motor neuron

Answers
For reflex action the motor neuron that generates the reflex action and pass to the sensory neuron. Interneuron helps to sensory neuron to communicate with motor neurons. Thus in this picture, the motor neuron be the interneuron and interneuron be the motor neuron. Thus option A and D are correct.neuron should be the interneuron.
What is reflex action?Reflex action is generated by our body by the sudden action to for sense of touch or other stimulants. For example, if touch a very hot body, we will take our hand within second and this is resulted by the action of neuron and is called the reflex action.
The movement of hands and legs are controlled by the motor neurons. If we touch any stimulant sensory neurons pass signals to the motor neuron and a signal containing the information of action is returned to the sensory neurons of our hand.
Here, the communication between sensory neuron and motor neuron is facilitated by interneurons. Thus, in this picture the motor neuron be the interneuron and interneuron be the motor neuron. Thus option A and D are correct.
To find more on reflex action, refer here:
http://brainly.com/question/4570017
#SPJ1
The pH of an aqueous solution _________
A. is not affected by added OH
B. depends solely on the ionization of water.
C. is a function of hydrogen ion concentration (to a reasonable approximation)
D. must remain at 7
Answers
Answer:
(B) depends solely on the ionization of water.
Explanation: hope this helps !
In plant cells, chloroplasts ...
A. Support and protect the cell.
B. Allow materials to move into and out of the cell.
C. Helps plant cells to produce their own food (glucose).
D. Makes the cell energy
Answers
hope that helps !!
Match the element with its number of valence electrons.
Column A
Column B
Helium
h
2.
Aluminum
b. 3
3.
Fluorine
C. 2
4.
Sodium
Answers
Complete Question:
Match the element with its number of valence electrons.
Column A
1. Helium.
2. Aluminum.
3. Fluorine.
4. Sodium.
Column B
A. 7
B. 1
C. 3
D. 2
Answer:
1. D
2. C
3. A
4. B
Explanation:
In Chemistry, valence electrons can be defined as the number of electrons present in the outermost shell of an atom. Valence electrons are used to determine whether an atom or group of elements found in a periodic table can bond with others. Thus, this property is typically used to determine the chemical properties of elements.
1. Helium: this element is found in group (2) of the periodic table and as such it has 2 electrons in its outermost shell. Therefore, Helium has two (2) valence electrons.
2. Aluminum: it is an element found in group (3) of the periodic table and as such it has 3 electrons in its outermost shell. Therefore, Aluminum has three (3) valence electrons.
3. Fluorine: it is found in group (7) of the periodic table known as halogens and as such it has 7 electrons in its outermost shell. Therefore, Fluorine has seven (7) valence electrons.
4. Sodium: this element is found in group (1) of the periodic table and as such it has 1 electrons in its outermost shell. Hence, Sodium has two (1) valence electrons.
Based on a Kc value of 0.250 and the given data table, what are the equilibrium concentrations of XY, X, and Y , respectively?
Answers
From the solution that we have in the question;
The concentration of X and Y is 0.28 MThe concentration of XY is 0.32 MWhat is the equilibrium constant?The equilibrium constant, denoted as K, is a value that quantitatively represents the ratio of the concentrations of products to reactants at equilibrium in a chemical reaction.
It is a fundamental concept in chemical equilibrium.
The value of the equilibrium constant provides valuable information about the position of equilibrium and the relative concentrations of species involved in a chemical reaction.
Kc = [X] [Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\)
\(0.25(0.5 - x) = (0.1 + x)^2\)
\(0.125 - 0.25x =0.01 + 0.2x + x^2\\ x^2 + 0.45x - 0.115 = 0\)
x = 0.18 M
The equilibrium amount of X and Y= 0.28 M and the equilibrium concentration of XY = 0.32 M
Learn more about equilibrium constant:
https://brainly.com/question/29253884
#SPJ1
Based on the answer to the question that we have;
A 0.28 M concentration of X and Y exists at equilibriumXY's concentration at equilibrium is 0.32 M.The equilibrium constantThe ratio of the product to reactant concentrations in a chemical reaction at equilibrium is represented quantitatively by the equilibrium constant, abbreviated as K.
It is a cornerstone of the theory of chemical equilibrium.
A chemical reaction's equilibrium position and the relative concentrations of the species involved can both be learned from the equilibrium constant's value.
Kc = [X][Y]/[XY]
\(0.25 = (0.1 + x)^2/(0.5 - x)\\0.25(0.5 - x) = (0.1 +x)^2\\0.125 - 0.25x = 0.01 +0.2x +x^2\\= 0.18 M\)
The equilibrium concentration of;
XY =0.5 - 0.18
=0.32 M
Then the equilibrium amount of
X and Y is
0.1 + 0.18= 0.28 M.
Learn more about equilibrium:brainly.com/question/29253884
#SPJ1

pls help
Which subatomic particle has a neutral charge?
A) electron
B) neutron
C) nucleus
D) proton
Answers
Answer: B
Neutrons are a combination of electrons and protons. They do not have a charge because of this reason. Protons and electrons have positive and negative charges.
Hope it helps!
what happens to a circuits resistance (R), voltage (V), and current (I) when you increase the diameter of the wire in the circuit?
a. R increases, V is constant, I increases
b. R decreases, V is constant, I increases
c. R decreases, V increases, I increases
d. R increases, V decreases, I decreases
Answers
Answer:
b. R decreases, V is constant, I increases
Explanation:
when we increase the diameter of wire increases ,resistance decreases and current increases.Therefore, the option is b.
Resistance is an electrical quantity which opposes the electric current in the circuit. Resistance is directly proportional to the length of the wire and inversely proportional to the diameter of the wire. If length of wire increases resistance will increases and if the diameter of the wire increase resistance will decreases.
R = ρ L/A
Here ρ is the resistivity
L is the length of the wire
A is the cross-sectional area of wire/diameter of the wire
Voltage is the potential difference between two terminals of voltage source. Current is the flow of electrons in the circuit. Voltage is the product of current and resistance.
V=IR
Rewrite the above equation interms of current
I=V/R
From the above equation we can say that current is inversly proportional to the resistance.Therefore,the correct option is b.
Learn more about,resistance
https://brainly.com/question/4289257
A saline solution, NaCl in water, is 0.92 % (m/v). How many grams of NaCl are required to prepare 35.0 mL of this solution?
Answers
Answer:
0.322 g
Explanation:
Since our concentration is given in mass per volume percent % (m/v)
% (m/v) = mass of solute in g/volume of solution in mL × 100%
Since % (m/v) = 0.92 and volume of the required solution is 35.0 mL, we find the mass of NaCl from
mass of NaCl = % (m/v)/100 % × volume of solution
= 0.92 % (m/v)/100 %× 35 mL
= 0.0092 × 35
= 0.322 g
The study of a chemical is called chemistry.
The correct answer is 0.322 g
The saline is said that the amount of salt present in the solution. The formula used to solve the question is as follows:-
\(\frac{m}{v} = \frac{mass \ of\ solute}{volume\ of\ solution} * 100\)
The data is given as follows:-
0.92 %(m/v)Volume is 35.0 mL,Mass of NaCl is as follows:-
\(\frac{0.92}{100} * 35 mL\)
\(0.0092 * 35\\= 0.322 g\)
Hence, the correct answer is 0.322g.
For more information, refer to the link:-
https://brainly.com/question/14698383
Is 2B+3Cl2->BCl3 a redox reaction?
Answers
The mass spectra of alcohols often fail to exhibit detectable M peaks but instead show relatively large _____ peaks?A) M+1
B) M+2
C) M-16
D) M-17
E) M-18
Answers
Answer:
E) M-18
due to the loss of water
What is the boiling point in °C of a 3.6 molal solution of calcium chloride in water?

Answers
Answer:
CaCl2--->Ca2+ + 2Cl_ so i=3
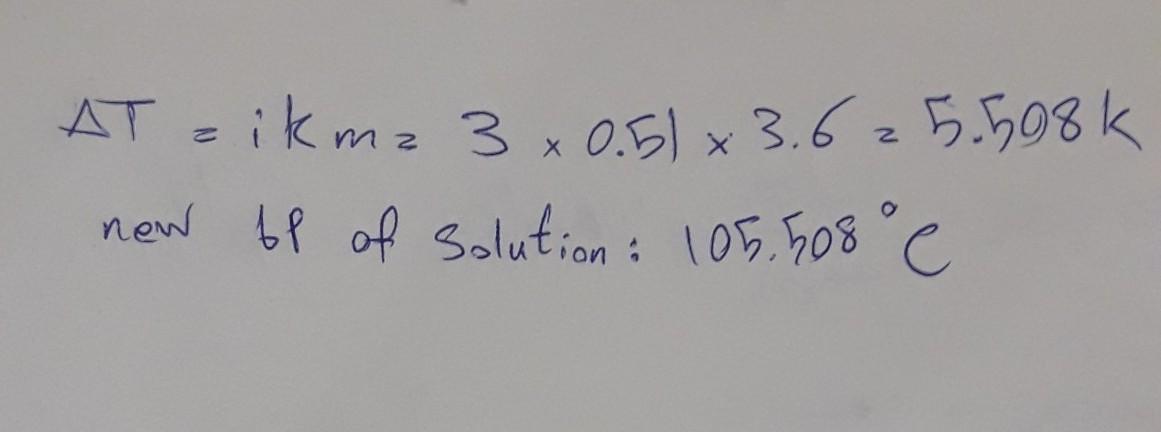
A 1.0 mole sample of fluorine gas at 25 °C has an average molecular velocity of 415 m/s. What is the total KE of the gas sample? Report your answer in kilojoules to the nearest tenth.
Answers
The total kinetic energy of the gas sample is 3.3 KJ
What is kinetic energy?This is the energy possessed by an object in motion. Mathematically, it can be expressed as:
KE = ½mv²
Where
KE is the kinetic energy m is the mass v is the velocity How to determine the mass of the fluorine gasMolar mass of fluorine gas = 38 g/molMole of fluorine gas = 1 moleMass of fluorine gas = ?Mass = mole × molar mass
Mass of fluorine gas = 1 × 38
Mass of fluorine gas = 38 g
How to determine the KE of the gas sampleMass (m) = 38 g = 38 / 1000 = 0.038 KgVelocity (v) = 415 m/sKinetic energy (KE) =?KE = ½mv²
KE = ½ × 0.038 × 415²
KE = 3272.275 J
Divide by 1000 to express in kilojoule
KE = 3272.275 / 1000
KE = 3.3 KJ
Learn more about energy:
https://brainly.com/question/10703928
#SPJ1
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
Hey....Tell me
What is the molecular weight of carbon dioxide??
follow me....
Answers
Answer:
44 g/mol
Explanation:
Draw a mechanism for the reaction of methylamine with 2-methylpropanoic acid. Draw any necessary curved arrows. Show the products of the reaction. Include any nonzero formal charges and all lone pairs of electrons. Indicate which side of the reaction is favored at equilibrium.
Answers
Answer:
See figure 1
Explanation:
On this case we have a base (methylamine) and an acid (2-methyl propanoic acid). When we have an acid and a base an acid-base reaction will take place, on this specific case we will produce an ammonium carboxylate salt.
Now the question is: ¿These compounds can react by a nucleophile acyl substitution reaction? in other words ¿These compounds can produce an amide?
Due to the nature of the compounds (base and acid), the nucleophile (methylamine) doesn't have the ability to attack the carbon of the carbonyl group due to his basicity. The methylamine will react with the acid-producing a positive charge on the nitrogen and with this charge, the methylamine loses all his nucleophilicity.
I hope it helps!

What is the reducing agent in the redox reaction represented by the following cell notation?
Zn(s) ∣ Zn2+(aq) ∣∣ Ag+(aq) ∣ Ag(s)
Answers
To solve such this we must know the concept of redox reaction. Therefore, Zn is the reducing agent represented by the given cell notation
Zn(s) ∣ Zn2+(aq) ∣∣ Ag+(aq) ∣ Ag(s)
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction.
Redox reaction is a chemical reaction where oxidation and reduction takes place simultaneously. Oxidation is loss of electrons and reduction is gain of electrons. The electron transfer from oxidant to reductant. Zn is the reducing agent in the given reaction. The component that undergoes oxidation is known as a reducing agent.
Therefore, Zn is the reducing agent in the given reaction.
Learn more about the chemical reactions, here:
brainly.com/question/3461108
#SPJ5
In which region is the substance in both the solid phase and the liquid phase? PLS HURRY

Answers
Answer:
2
Explanation:
At the constant temp, the substance is going from a solid to a liquid and both are present (like melting ice)
Convert 57.0 degrees C todegrees F.[?] °FEnterT in degrees F
![Convert 57.0 degrees C todegrees F.[?] FEnterT in degrees F](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/aAShLUZ6J8jn13E9pm2XDAAgB3OdgPxq.jpeg)
Answers
Answer
134.6 °F
Explanation
To covert degrees C to degrees F use:
(0°C × 9/5) + 32 = 32°F
Therefore 0 °C = 32 °F
We are given 57.0 °C
(57.0°C x 9/5) + 32 = 134.6 °F
Generally, how do atomic masses vary throughout the periodic table of the elements?
They increase from left to right and top to bottom.
O They increase from left to right and bottom to top.
O They increase from right to left and top to bottom.
O They increase from right to left and bottom to top.
Answers
Answer:
They increase from left to right and top to bottom.
Answer:
They increase from left to right and top to bottom.
90Sr →?+e
38
Express your answer as a nuclear equation
Answers
Strontium ( Atomic Number 38 ) undergoes beta minus decay. Atomic mass of Strontium is 90 u. The given reaction shows the complete nuclear reaction of Strontium.
⁹⁰₃₈ Sr → ⁹⁰₃₇ Y + ⁰₋₁ β + ⁻∨ₑ
Beta particle β is a high speed electron. Whenever Strontium undergoes beta minus decay, the neutron located inside its nucleus gets converted into proton. During the same reaction, a beta particle and electron antineutrino are also being released or emitted.
In the nuclear reaction ( Beta minus decay ) of Strontium, mass and charge are conserved.
Learn more about Nuclear Reactions here:
https://brainly.com/question/12739650
#SPJ9