Which statements describe kinetic and potential energy? Check all that apply
Answers
umm is there an answer choices that go with this????
Related Questions
> A Moving to another question will save this response. Question 1 What is the mass number of an atom of potassium that has 20 neutrons?
a. 35
b. 59
c. 39
d. 15
e. 19
Answers
The mass number of an atom of potassium that has 20 neutrons is option C. 39
Mass number-
Mass number is the total number of protons and neutrons in the nucleus of an atom is called the mass number. It is represented by the symbol A. In other words, mass number refers to the sum of the number of protons and neutrons in the nucleus of an atom.
Atom-
Atoms are tiny particles that make up everything in the world. Everything in the world is made up of tiny particles known as atoms. An atom is the basic unit of matter. The term "atom" comes from the Greek word atomos, which means indivisible.
The basic building blocks of all matter are atoms, which are made up of three types of particles: protons, neutrons, and electrons.
In a neutral atom, the number of electrons is equal to the number of protons, thus the overall charge on the atom is zero. However, the mass number of the atom is equal to the sum of the number of protons and neutrons.
Learn more about the mass number of an atom from the given link-
https://brainly.com/question/26160840
#SPJ11
what is the molecular polarity of the following lewis structure

Answers
Help please i am being timed

Answers
Answer:
b
Explanation:
got it wrong on edge2020
Answer:
its c because the northern and southern hemisphere get the same amount of energy from the son in march
Structures and Forces - What is force and external forces.
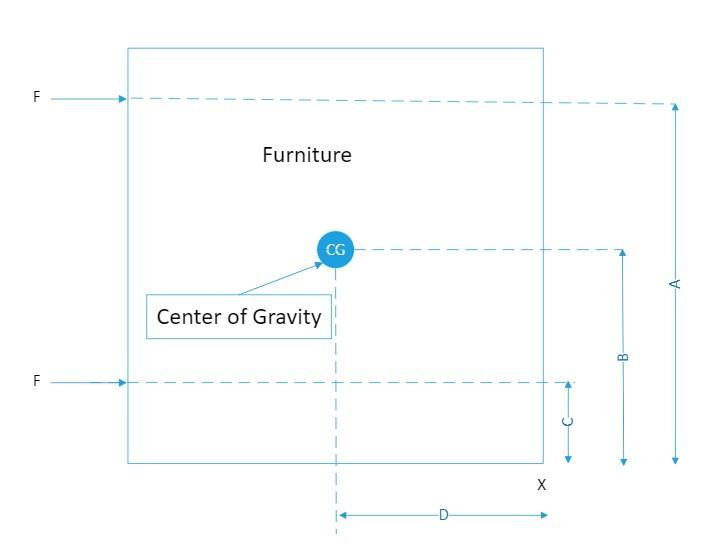
Question 1) Jacob and Ravleen are moving furniture. They need to move the filing cabinet over 6 feet and it is very heavy. Jacob wants to apply force and push it by putting his hands on point A because he thinks it would be easier to move it from the top as there is less weight on the top. Ravleen says it's better to push at point B because the center of gravity is lower. Which person is correct and EXPLAIN why and what may happen if it is pushed in the wrong spot.
Answers
Answer:
The restoring force that prevents the furniture from tipping over when pushing the furniture towards the right, F= W × D
Where;
W = The weight of the furniture
D = The horizontal distance from the center of gravity of the furniture, CG and the to the lower right corner of the furniture
Given that A > D, for the furniture to tip over when pushing above the CG, we have;
F1 × A ≤ W × D
∴ F1 << W or for a force much smaller than W, the weight of the furniture applied above the center of gravity, the furniture will tip over
However when the force F2 is applied, where C < D, we have for no tipping over;
F2 × C ≤ W × D, therefore, given that C < D, when a force much higher than the weight, W, is applied at F2, the furniture will remain upright
Therefore, it is better push at a point lower than or equal to the center of gravity, whereby the point B is lower than the center of gravity, it is better to push at point B, Ravleen is correct and it is better to push at point B because the center of gravity is lower
If the furniture is pushed at the wrong spot it may tip over
Please see attached drawing
Explanation:
What information is provided by the specific block location of an element?.
Answers
The block location of an element tells the energy sublevel of an element.
What is block location of an element?The term block location refers to the specific block in which an element is found in the periodic table. There are three blocks in the periodic table which are;
s - blockp - blockd - block and f - blockThe block location of an element tells the energy sublevel of an element.
Learn more about blocks:https://brainly.com/question/3580092
100 PTS IM DESPERATE NEED BY TOMMOROW


Answers
Answer:
rest will do later mom is calling me plz understand promise will do it
Explanation:
Elements and compounds are similar in that they are both made of atoms and in some cases molecules.Methods of Breaking Down CompoundsThe only way to break down a compound is through a chemical change. Sometimes, energy is needed for a chemical change to happen. Two ways to add energy to break down a compound are to apply heat and to apply an electric current.Answer:
lol
Explanation:
its 5 points
All living organisms are composed of
I need help on this, i'll give 30 pts for brainliest
Answers
Answer:
All living organisms are made up of one or more cells, which are considered the fundamental units of life. Even unicellular organisms are complex! Inside each cell, atoms make up molecules, which make up cell organelles and structures. In multicellular organisms, similar cells form tissues.
Explanation:
Answer:
All living organisms are composed of one or more cell
Explanation:
hope this helps, please mark brainliest
what mass of nacl is in 2.5L of 0.207M solution?
Answers
Pls mark brainlest.!:)
Which of the following contains factors that all affect polymer properties? A. chain length, intermolecular forces, and the molar mass of the monomers B. chain length, the C-H bond strength, and the extent of chain branchingC. chain length, intermolecular forces, and the strength of C- H bonds D. chain length, intermolecular forces, and the extent of chain branching
Answers
The answer is D. Chain length, intermolecular forces, and the extent of chain branching all affect polymer properties.
Chain length influences a polymer's mechanical and physical properties, such as tensile strength and toughness. As the chain length increases, the polymer becomes more resistant to deformation. Intermolecular forces, including van der Waals forces, hydrogen bonding, and dipole-dipole interactions, play a crucial role in determining a polymer's thermal, mechanical, and solubility properties. Polymers with strong intermolecular forces exhibit higher melting points and increased mechanical strength.
Finally, the extent of chain branching impacts a polymer's properties by influencing its crystallinity, density, and molecular weight distribution. Highly branched polymers have lower crystallinity and density, which can result in reduced mechanical strength and increased permeability. Understanding these factors is essential for designing polymers with specific desired properties for various applications.
Learn more about polymers here:
https://brainly.com/question/1443134
#SPJ11
Which name is given to a reaction in which the number of each type of atom on one side of the equation is equal to the number of the same kind of atoms on the other side of the equation?
Answers
A balanced chemical equation describes a reaction where the number of each type of atom on one side of the equation is equal to the number of the same sort of atoms on the other side of the equation.
In other words, the number of atoms of each element is the same on both sides of the equation, obeying the rule of conservation of mass.
For instance, the balanced formula for the combustion of oxygen and methane is CH4 + 2 O2 -> CO2 + 2 H2O.
On either side of the equation, there are 1 carbon atom, 4 hydrogen atoms, and 4 oxygen atoms.
The chemical equation must be in balance in order to employ the right quantity of reactants and products and to forecast how much reactant will be used and how much product will be produced.
learn more about atoms here:
https://brainly.com/question/1566330
#SPJ4
(50 points help ASAP it’s due 10mins)About how much more TE does it take to change the temperature of 1 kg of water compared to 1 kg of iron?
A. 5:L1
B. 12:1
C. 9:1
D. 2:1
Answers
Answer:c
Explanation:
Write the net ionic equation of HNO3+Fe--> Fe(HNO3)2 +H2
Answers
Answer:
nitric acid + iron=iron+[nitric acid ] 2 hydrogen
The net ionic equation will be Fe → \(Fe^{2+}\).
What is net ionic equation?The net ionic equation would be a chemical equation which only indicates the elements, compounds, as well as ions instantly involved in a chemical reaction.
The given chemical equation is:
HNO3+Fe → Fe(HNO3)2 +H2
It can be written in term of ionic equation.
\(H^{+}\) + \(NO_{3}^{-}\)+Fe → \(Fe^{2+}\) + \(H^{+}\) + \(NO_{3}^{-}\)
Hydrogen ion and nitrate ion will be canceled each other.
Hence, the net ionic equation will be
Fe → \(Fe^{2+}\)
To know more about net ionic equation.
https://brainly.com/question/22885959
#SPJ2
Chemistry students were testing compounds in an aqueous solution to see which one had the strongest electrical current. There are three beakers each with a different compound. The first beaker and the second beaker lit the light bulbs brightly. The third beaker did not light up the bulb. With the information that is provided, what is the best explanation for the first and second beaker being the strongest electrolytes?
A)The first and second beakers contain a solution that partially dissociates the ions. These ions create an electrical current. The third beaker solution completely dissociates the ions. It is a strong electrolyte.
B)The first and second beakers contain a solution that completely dissociates the ions. These ions create an electrical current. The third beaker solution does not dissociate ions. It is a nonelectrolyte.
C)The first and second beakers contain a solution that does not dissociate ions. These ions create an electrical current. The third beaker solution completely dissociates the ions. It is a strong electrolyte.
D)The first and second beakers contain a solution that completely dissociates the ions. These ions do not create an electrical current. The third beaker solution does completely dissociates the ions. It is a strong electrolyte.
Answers
Answer:
B)The first and second beakers contain a solution that completely dissociates the ions. These ions create an electrical current. The third beaker solution does not dissociate ions. It is a nonelectrolyte.
Explanation:
Electrolytes dissociate ions. They are also conductors. Since the first 2 bulbs light up strongly, we can assume that the ions completely dissociated. Since the 3rd did not light up, it is a nonelectrolyte.
Answer:
The first and second beakers contain a solution that completely dissociates the ions. These ions create an electrical current. The third beaker solution does not dissociate ions. It is a nonelectrolyte.
Explanation:
assume vinegar is a 0.852 m solution of acetic acid (hc2h3o2) in water. what volume of 0.214 m naoh would be needed to completely neutralize 5.26 ml of vinegar?
Answers
According to the question, the given solution is vinegar, which is a 0.852-molar solution of acetic acid (HC2H3O2) in water. The volume of NaOH needed to neutralize 5.26 mL of vinegar is explained below .
To solve the problem, we will use the concept of the neutralization reaction of acetic acid and NaOH.
Let us write the balanced chemical equation for the reaction:
HC2H3O2 + NaOH → NaC2H3O2 + H2O
From the equation, we can see that 1 mole of HC2H3O2 reacts with 1 mole of NaOH. The molarity of acetic acid is given as 0.852 M, which means that 1 L of the solution contains 0.852 moles of acetic acid. Therefore, the number of moles of acetic acid in 5.26 mL of vinegar can be calculated as follows:
Number of moles of acetic acid = Molarity × Volume = 0.852 M × (5.26 mL / 1000 mL/L) = 0.00449 moles
Since the stoichiometry of the reaction is 1:1, we need an equal number of moles of NaOH to neutralize the acetic acid. The molarity of NaOH is given as 0.214 M, which means that 1 L of the solution contains 0.214 moles of NaOH.
Therefore, the volume of NaOH needed can be calculated as follows:
Volume of NaOH = Number of moles of NaOH / Molarity = 0.00449 moles / 0.214 M = 0.02098 L
Therefore, the volume of NaOH needed to completely neutralize 5.26 mL of vinegar is 0.02098 L or 20.98 mL.
To know more about the NaOH https://brainly.com/question/30232518
#SPJ11
help with this question please
A gas exerts a pressure of 1956 mm Hg on the wall of its container. what is this quantity in Pascals? (760.00 mm Hg=101.325 kPa)
Answers
The frequency and intensity of gas-gas collisions are measured by gas pressure. 1 Atm is equivalent to 760 Torr, 760 mmHg, 101.325 kPa, 101,325 Pa, 14.7 lb/in2, and 29.92 inHg.
What kind of use is quantity?A Brief Glance at Quantity. When referring to a single word that CANNOT be measured, use the word amount. – When referring to the a plural or singular term that CAN be tallied, use number. – When speaking to an inanimate, singular or plural word that CAN be numbered or measured, quantity should be used.
What are the different forms of quantity?There are two types of physical quantities. Qualities might be derived or basic. Quantities that cannot be represented in terms of any physical quantities are referred to as foundational or base quantities. They are size, mass, time, and space.
To know more about Quantity visit:
https://brainly.com/question/12986460
#SPJ13
How many grams of magnesium (Mg) are required to produce 40.0 grams of boron (B)?
Answers
3.7 mol Mg
See image:
Hope this helps,
Jeron
:- )

24.3 g of magnesium is required for two moles of the product. . Therefore, 356.3 grams of magnesium (Mg) are required to produce 40.0 grams of boron (B) through the reaction
What is a balanced chemical equation ?A balanced chemical equation represents the correct stoichiometry of all the reactants and products.
Based on the balanced chemical equation above, 2 moles of Mg are required to produce 1 mole of B2H6, and 1 mole of B2H6 produces 2 moles of MgB2.
Using the molar masses of Mg (24.31 g/mol) and B (10.81 g/mol), we can calculate the mass of Mg required to produce 40.0 g of B:
1 mole of B = 2 moles of Mg x (24.31 g/mol) = 48.62 g of Mg
40.0 g of B x (1 mol B / 10.81 g) x (2 mol Mg / 1 mol B) x (24.31 g / 1 mol Mg) = 356.3 g of Mg
Therefore, 356.3 grams of magnesium (Mg) are required to produce 40.0 grams of boron (B) through the reaction .
Find more on balanced reactions:
https://brainly.com/question/14280002
#SPJ2
The reaction related to your question is :
Mg + B2H6 → 2 MgB2 + 3 H2.
Mention two substance that sublines
Answers
Familiar substances that sublime readily include iodine , dry ice , menthol, and camphor. Sublimation is occasionally used in the laboratory as a method for purification of solids, for example, with caffeine.
The 1H nucleus is composed of a single proton and therefore has a relative mass loss of 0 g/mol. Select the isotope deuterium, 2H. What is the relative mass loss of deuterium, 2H, in g/mol?
Answers
The nucleus of 1H is made up of a single proton and therefore has a relative mass loss of 0 g/mol. The deuterium isotope, 2H, on the other hand, has a relative mass loss of 2.014 g/mol. The mass number of 2H is two, indicating that it has one neutron and one proton in its nucleus. Let's look at some additional detail about isotopes: A given element has the same number of protons, but it can have different numbers of neutrons in its nucleus.
A different isotope of the same element is formed by adding or subtracting neutrons from the nucleus. Isotopes of the same element have the same number of protons but a different number of neutrons. The atomic mass of an element is calculated by summing the number of protons and neutrons in the nucleus. The atomic mass unit (amu) is the unit used to calculate atomic mass. In conclusion, The relative atomic mass of 2H is 2.014 g/mol, which is a loss of 0.014 g/mol relative to the mass of 2 protons. The relative mass loss of deuterium, 2H, is 0.014 g/mol.
To know more about atomic mass unit visit
https://brainly.com/question/8085884
#SPJ11
Cooking an egg is one type of process, while the formation of snow is another. In three to five sentences, identify each as exothermic or endothermic and explain how you know. (4 points)
Answers
An omelet is endothermic because it absorbs heat from its surroundings when it is being baked. Since water dissipates hot air while forming snow, snow creation is exothermic. Egg frying is exothermic.
How to explain endothermic?Chemical processes known as endothermic reactions use heat from the surroundings to produce products. These occurrences have the effect of chilling their immediate surroundings, which results in a cooling effect.
Endothermic heat: what is it?If the heat is absorbed either by system from the environment, the reaction or states of matter is endothermic. As a result of the system absorbing heat from its surroundings during an endothermic cycle, the environment cools.
To know more about endothermic visit:
https://brainly.com/question/23184814
#SPJ1
(EASY POINTS!!!!!!!!!!!!!!!)How do we change the world... one random act of kindness at a time
Answers
Answer:
less polution
Explanation:
(5 points) For the Complex III in the electron transport chain:
Complex III step 1: UQH2 is oxidized in a 2 electron process. Cytochrome c is reduced and UQ is reduced to UQH in two 1 electron processes.
Complex III step 2: UQH2 is oxidized in a 2 electron process. Cytochrome c is reduced and UQH is reduced to UQH2 in two 1 electron processes.
The necessary standard reduction potentials are:
UQ + 2H+ + 2e- UQH2 E° = 0.06 V
cyt c (Fe3+) + e- cyt c (Fe2+) E° = 0.254 V
UQ + H+ + e- UQH. E° = 0.03 V
UQH. + H+ + e- UQH2 E° = 0.19 V
Calculate the total redox potential of the complex.
(5 Points) Now calculate how many moles of protons can be translocated across the inner mitochondrial membrane if translocation of 1 mole requires 23 kJ.
(5 Points) Calculate the free energy available for proton translocation assuming a 2electron process for each complex.
Answers
Answer:
Step 1:
UQH2 + 2 cyt c (Fe3+) → UQ + 2 cyt c (Fe2+)
This step involves the oxidation of UQH2 and reduction of cyt c (Fe3+). The net reaction involves a 2-electron transfer from UQH2 to cyt c (Fe3+).
The standard reduction potential for UQH2 to UQ is given as 0.06 V, and for cyt c (Fe3+) to cyt c (Fe2+) it is 0.254 V.
The net standard reduction potential for step 1 can be calculated as follows:
E°_net1 = E°(UQH2) - E°(cyt c (Fe3+))
E°_net1 = 0.06 V - 0.254 V
E°_net1 = -0.194 V
Step 2:
UQH2 + 2 cyt c (Fe3+) → UQH + 2 cyt c (Fe2+)
This step also involves the oxidation of UQH2 and reduction of cyt c (Fe3+). The net reaction involves a 2-electron transfer from UQH2 to cyt c (Fe3+).
The standard reduction potential for UQH2 to UQH is given as 0.19 V.
The net standard reduction potential for step 2 can be calculated as follows:
E°_net2 = E°(UQH2) - E°(cyt c (Fe3+))
E°_net2 = 0.19 V - 0.254 V
E°_net2 = -0.064 V
Total redox potential of Complex III:
To calculate the total redox potential, we sum up the net reduction potentials of step 1 and step 2:
E°_total = E°_net1 + E°_net2
E°_total = -0.194 V + (-0.064 V)
E°_total = -0.258 V
Now, let's calculate the free energy available for proton translocation assuming a 2-electron process for each complex.
The equation relating free energy change (ΔG) and standard reduction potential (E°) is given by:
ΔG = -nFΔE°
Where:
ΔG is the free energy change
n is the number of electrons transferred
F is Faraday's constant (96,485 C/mol)
ΔE° is the standard reduction potential
For a 2-electron process, n = 2.
ΔG1 = -2 * 96,485 C/mol * (-0.194 V)
ΔG1 = 37,508.12 J/mol
ΔG2 = -2 * 96,485 C/mol * (-0.064 V)
ΔG2 = 12,303.04 J/mol
Therefore, the free energy available for proton translocation for each complex is 37,508.12 J/mol for Complex III, step 1, and 12,303.04 J/mol for Complex III, step 2.
To calculate the moles of protons translocated, we can use the equation:
ΔG = nFΔp
Where:
ΔG is the free energy change in joules
n is the number of moles of protons
F is Faraday's constant (96,485 C/mol)
Δp is the potential difference finish up now
Question 13 of 25
Choose the polar molecule.
о A. H2O
OB. C₂H4
O C. CO₂
OD. Cl₂
Answers
According to the molecular geometry, water is a polar molecule among the following.
What is molecular geometry?Molecular geometry can be defined as a three -dimensional arrangement of atoms which constitute the molecule.It includes parameters like bond length,bond angle and torsional angles.
It influences many properties of molecules like reactivity,polarity color,magnetism .The molecular geometry can be determined by various spectroscopic methods and diffraction methods , some of which are infrared,microwave and Raman spectroscopy.
They provide information about geometry by taking into considerations the vibrational and rotational absorbance of a substance.Neutron and electron diffraction techniques provide information about the distance between nuclei and electron density.
Learn more about molecular geometry,here:
https://brainly.com/question/30185738
#SPJ9
In the titration of a weak acid with a strong base, the point where half of the acid has reacted with the base is noteworthy because the ph equals:__________
Answers
In the titration of a weak acid with a strong base, the point where half of the acid has reacted with the base is noteworthy because the ph equals: the pKa.
What is pKa ?In layman's terms, pKa is a measurement of an acid's strength. A strong acid will have a pKa value that is lower than 0. To be more specific, pKa is the Ka value's negative log base ten value (acid dissociation constant). It gauges an acid's potency by determining how firmly a proton is retained by a Bronsted acid.
The strength of the acid and its capacity to donate protons increase with decreasing pKa values.
Acid dissociation constant, Ka, gauges how well an acid separates from its water-soluble constituents. Since acid generally dissociates into its ions, the stronger the acid, the higher the value of Ka.
The following equation describes the connection between pKa and Ka:
pKa = –log[Ka]
To view more about acids and bases, refer to:
https://brainly.com/question/11510137
#SPJ4
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
Situation B would produce what kind of spectra?
Hot Gas
Cold Gas
help

Answers
Answer:
It would be C because its continuing to the next. It doesn't stop nor does it absorb anything.
Explanation: I hope this helps :) I'm only in 8th grade but this seems easy so if I am wrong I am soo sorry.
What is the name of this hydrocarbon? a skeletal model has 2 central carbons bonded to c h 3 at each end, and at the left of the 2 central carbons. 3-isopentane 2-methylbutane 3-methylbutane 1,2-methylpropane
Answers
The name of the given hydrocarbon in which 2 central carbon atoms with additional 3 methyl groups is 2-methylbutane.
What are hydrocarbons?Hydrocarbons are the organic compounds which are made up of by the combination of hydrogen and carbon atoms.
According to the question desired structure is attached below where:
2 and 3 number shows the central carbon atoms which are present in the hydrocarbon.1 and 4 number shows the methyl groups that are attached at each end.One extra methyl group is attach on the left side of 2nd central carbon atoms.Hence required hydrocarbon is 2-methyl butane.
To know more about hydrocarbons, visit the below link:
https://brainly.com/question/3551546

Answer:
B. 2-methylbutane
Explanation:
Correct on Edge 2022!!!
Good luck everyone, you got this! Have a great day!
how many atoms make up a molecule of ethanol c2h6o
Answers
A molecule of ethanol (C2H6O) is composed of 9 atoms.
Breaking down the molecular formula: C2H6O
There are 2 carbon atoms (C2).
There are 6 hydrogen atoms (H6).
There is 1 oxygen atom (O).
In total, the molecule of ethanol contains 2 carbon atoms, 6 hydrogen atoms, and 1 oxygen atom, summing up to a total of 9 atoms.
A molecule of ethanol (C2H6O) consists of 2 carbon atoms (C), 6 hydrogen atoms (H), and 1 oxygen atom (O). Therefore, there are a total of 2 + 6 + 1 = 9 atoms in a molecule of ethanol.
Adding up the individual atoms, we get a total of 2 carbon atoms, 6 hydrogen atoms, and 1 oxygen atom, which sum up to 9 atoms in total.
To learn more about atoms
https://brainly.com/question/17545314
#SPJ11
A thermite reaction releases large amounts of heat and light, resulting in the melting of the iron metal that forms during the reaction.
fe2o3 + 2al → al2o3 + 2fe
determine the correct mole ratio for iron (iii) oxide and aluminum based on the balanced chemical equation. fe2o3:al =
Answers
Answer:
The mole ratio for iron (iii) oxide to aluminium is
1:2
Which type of molecule is octanal?
A. Alcohol
B. Ketone
C. Amine
D. Aldehyde
Answers
the ending (al) tells you that. alcohols end in “ol”, ketones end in “one”, amines have 2 different ways of naming so i suggest finding which one you want to use, and aldehyde end in “al” :)
gold is fcc and has a lattice constant of 0.40788 nm. calculate a value for the atomic radius of a gold atom in nanometers.
Answers
Gold is fcc and has a lattice constant of 0.40788 nm . The value for the atomic radius of a gold atom in nanometers is 0.14421 nm. .
A gold atom touches another along the diagonal on each face of the unit cell of a fcc lattice, which has right angles. Typically, the diagonal is shown as extending from the center of one atom to the center of a second atom and to the center of a third atom. As a result, one can construct a right triangle whose hypotenuse is 4r, where r is the radius of a gold atom, and whose legs both have lengths of a = 0.40788 nm. By applying the Pythagorean theorem,
a^2 + a^2 = ( 4r )^2,
2 a^2 + = (16r^2) ,
and r = (21/2/4)a,
where an is the stated covalent atomic radius of gold and r is 0.14421 nm.
learn about atomic radius
https://brainly.com/question/13607061
#SPJ4