Which particle has two neutrons?
o 1 0 n
O 2 1 H
o 4 2 He
O 1 1 H
Answers
Related Questions
what is the goal of the nifty home experiment
Answers
A playing card was placed on top of a glass during the clever at-home experiment, and then a quarter was placed on top of the card. The quarter landed in the glass when the card was pushed.
To determine the likelihood that the fetus will be born with specific chromosomal abnormalities, pregnant women can take the non-invasive prenatal screening test NIFTY (also known as cell-free DNA screening).
Because of its inertia, the coin is strongly motivated to remain stationary. The card cannot be moved quickly enough to defeat that force if it is moved slowly. The coin drops into the cup after staying in one position if you flick it swiftly.
To know more about nifty home experiments, visit;
https://brainly.com/question/35551719
#SPJ3
Raw sulfur containing 88% S and 12% inerts was burned in dry air supplied in 54% in excess (S to SO2). The cinder contained 6% S and the rest inerts. It was found that 79% of the S that was gasified burned to SO2 and the rest to SO3. Calculate the following
Percent excess air based on the complete conversion of S to SO3
Weight of cinder in kg
Amount of sulfur gasified in kmol
Percent of SO2 in the burner gas based on complete analysis
Answers
The sulfur in raw sulfur, which has an 88% S and 12% inerts composition, was burned in dry air supplied with 54% in excess. The cinder contained 6% S and the remaining inerts. It was discovered that 79% of the S that was gasified burned to SO2, and the remaining to SO3. The following are the calculations.
Percent excess air based on complete conversion of S to SO3S + 3O2 → SO3The equation shows that there is no excess air in the case of complete conversion of sulfur to SO3. The balanced equation indicates that 1 kmol of sulfur reacts with 3 kmol of oxygen to produce 1 kmol of SO3. This implies that 1.5 kmol of oxygen are necessary per kmol of S.
Given the air-supplying 54% in excess of S to SO2, the oxygen supply was 1.54(1.5 kmol O2/kmol S) = 2.31 kmol O2/kmol S.
The excess air required for the complete conversion of S to SO3 was thus (2.31 - 1.5)/1.5 × 100 = 54%.
The weight of cinder in kg
Cinder composition includes 6% sulfur. The amount of sulfur in the cinder is calculated as follows:
100 kg of cinder contains 6 kg of sulfur.
Mass of sulfur = Mass fraction × Total mass
= 6/100 × Total mass= 0.06
Total mass = Mass of sulfur/Mass fraction= 0.06/0.06= 1 kg
Amount of sulfur gasified in kmol
The weight of the sulfur present in the raw sulfur is calculated first: 100 kg of raw sulfur contains 88 kg of sulfur
Mass of sulfur = Mass fraction × Total mass= 88/100 × Total mass= 0.88
Total mass = Mass of sulfur/Mass fraction= 0.88/0.88= 1 kmol
SO2 percent in the burner gas based on complete analysis
The air needed to completely burn 1 kmol of sulfur to SO2 is 1 kmol S + 1.5 kmol O2 → 1 kmol SO2.
54% excess air was used in this situation, therefore, oxygen supply was 1.54(1.5 kmol O2/kmol S) = 2.31 kmol O2/kmol S. This implies that the air/fuel ratio was 2.31 kmol O2/kmol S. Since oxygen accounts for around 23 percent of the air, this equates to an air-fuel ratio of approximately 10 kmol air/kmol S. SO2 accounts for 20 percent of the dry products by volume. This equates to an SO2 concentration of 20/(20 + 80) = 20 percent by volume.
To know more about sulfur visit:
https://brainly.com/question/31780887
#SPJ11
Use the image above (Onion root tip mitosis). Which step of Mitosis does the letter (a) represent?

Answers
Answer:
Metaphase
Explanation:
Metaphase is characterized by the chromosomes lining up at the equator; they are getting ready to separate. As it looks like from the picture, it looks like the chromosomes are in the middle. The chromosomes aren't quite pulled apart. I'm going from visual judgment.
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law?
- High pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker
- High pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant
- Low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker
- Low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers
Answer:
High pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant
Answer:
A gas at very low volumes, when gas particles are very close together
A gas at very low temperatures, when gas particles have very little kinetic energy
A gas with highly polar molecules that have very strong inter-molecular forces
Explanation:
According to kinetic molecular theory:
a) Ideal gases have large number of particles which were considered to be hard spherical objects.
b) these gas particles move randomly in a straight line.
c) the path of gas particles change when they collide with each other or walls of vessel.
d) there is no or negligible inter molecular interactions between the gas particles.
e) the inter molecular distances is much higher than the size of particles.
f) the gas particles occupy negligible volume of the vessel.
g) during collision of gas particles is perfectly elastic.
h) the average kinetic energy of particles is constant at a constant temperature. It varies directly with the temperature.
What is the frequency of an x-ray
wave with an energy of
2.0 x 10^-17 J?
Please explain how!

Answers
Answer:
3.3 x 10⁻¹⁷ Hz
Explanation:
To find the frequency, you can use the following equation:
E = h / f
In this equation,
-----> E = energy (J)
-----> h = Planck's Constant (6.626 x 10⁻³⁴ J*s)
-----> f = frequency (Hz)
You can plug the given values into the equation and simplify to find the frequency. This equation will require a little bit of rearranging.
E = h / f <----- Given equation
(2.0 x 10⁻¹⁷ J) = (6.626 x 10⁻³⁴ J*s) / f <----- Insert values
(2.0 x 10⁻¹⁷ J) x f = (6.626 x 10⁻³⁴ J*s) <----- Multiply both sides by f
f = 3.3 x 10⁻¹⁷ Hz <----- Divide both sides by 2.0 x 10⁻¹⁷
The frequency of an x-ray wave with an energy of 2.0 x 10^-17 J is 3.3 x 10⁻¹⁷ Hz.
What is frequency ?
The term frequency is defined as the number of waves that pass a fixed point in unit time. Frequency is measured in hertz which is equal to one event per second.
Frequency also describes the number of cycles undergoes during one unit of time by a body in periodic motion.
Calculating the frequency, you can use the following equation:
E = h / f
Where,
E = energy (J)
h = Planck's Constant (6.626 x 10⁻³⁴ J*s)
f = frequency (Hz)
Insert his values in the given equation
(2.0 x 10⁻¹⁷ J) = (6.626 x 10⁻³⁴ J × s) / f
(2.0 x 10⁻¹⁷ J) x f = (6.626 x 10⁻³⁴ J × s)
f = 3.3 x 10⁻¹⁷ Hz
Thus, The frequency of an x-ray wave with an energy of 2.0 x 10^-17 J is 3.3 x 10⁻¹⁷ Hz.
To learn more about the frequency, follow the link;
https://brainly.com/question/5102661
#SPJ2
amino acids in the diet that are not used to make proteins can be used in __________. gluconeogenesis glycogenesis lipogenesis glycogenolysis
Answers
Amino acids in the diet that are not used to make proteins can be used in gluconeogenesis.
Gluconeogenesis is a metabolic pathway that occurs in the liver and kidneys, where certain amino acids can be converted into glucose. When the body has an excess of amino acids that are not needed for protein synthesis, they can be broken down and utilized to produce glucose molecules. Glucose is an essential energy source for the body and is needed for various metabolic processes. Gluconeogenesis helps maintain blood sugar levels and ensures a steady supply of glucose, especially during periods of fasting or when carbohydrate intake is insufficient.
In summary, amino acids in the diet that are not utilized for protein synthesis can be redirected and used in gluconeogenesis to generate glucose. This process provides an alternative pathway for amino acid metabolism and helps maintain energy balance and glucose homeostasis in the body.
To know more about Amino acids click here:
https://brainly.com/question/31872499
#SPJ11
Pls Help!
A sample of seawater from a tidal estuary was found to contain a concentration of 727 mg of chloride ion per kg of seawater. If the density of the sample was 1.035 g/mL, what is the molarity of the chloride ion?
Answers
The molarity of the chloride ion is 2.0 * 10⁻⁵ M.
What is the molarity of the chloride ion?The molarity of the chloride ion is calculated using the formula below:
Molarity = (Percentage concentration * Density ) / (Molar mass * 100)The percentage concentration of chloride ion = 727/kg * 10⁻⁶ Kg * 100/1
The percentage concentration of chloride ion = 0.0727%
Molar mass of Chloride ion = 35.5 g/mol
Molarity = (0.0727 * 1.035)/(35.5 * 100)
Molarity of chloride ion = 2.0 * 10⁻⁵ M
In conclusion, the molarity of the chloride ion is obtained from the density and percent concentration of chloride ions in seawater.
Learn more about molarity at: https://brainly.com/question/24305514
#SPJ1
the temperature will decrease the rate of reactions.
Answers
Answer:
true?
Explanation:
A ______ square is a diagram used to predict all possible allele combinations from a genetic ______. Using this diagram, the phenotypes of offspring can be determined from the genotypes.
Answers
Answer:
A Punnet Square...the second I am not sure.
Explanation:
Unfortunately the second could be many things. You'd have to have more background information.
Answer:
PUNNETT....,MAKE SURE U HAVE 2 T,s
Explanation:
punnett and cross
In the formation of SO2 and SO3 the ratio of the weight of oxygen which combines with 10kg of sulphur is ?
Answers
Explanation:
So,0.3125 * 10 ^3 moles of Sulphur combines with 0.3125 * 10^3 moles of Oxygen to from SO2. Therfore mass ratio of SO2 : SO3 = 10 : 15 = 2:3.
When an asteroid hit the Earth 65 million years ago, it threw a huge amount of dust into the atmosphere. This dust cloud stayed in the sky, blocking out the Sun for at least 10 years. Why did most of the animals on the Earth go extinct at this time?
A. The dust cloud prevented plants from making food
B. The asteroid started fires that burned the Earth
C. The Sun turned off
Answers
Answer:
A. i think
Explanation:
hope this helps u!
The dust cloud created by the asteroid impact caused a global climate change that drastically altered the Earth's environment. Hence, option A is correct.
What are the impacts of asteroids ?The dust and debris in the atmosphere blocked out the Sun's rays, causing a cooling effect on the planet's surface. This reduction in sunlight meant that photosynthesis, the process by which plants make food, was greatly reduced.
As a result, plant life was severely affected, leading to the extinction of many herbivorous animals that relied on them for food.
The loss of herbivores then caused a chain reaction of extinctions among carnivorous animals that relied on them for food. Additionally, the impact and resulting earthquakes and tsunamis, along with the massive fires that likely occurred, also contributed to the mass extinction event.
Find more on asteroids:
https://brainly.com/question/19161842
#SPJ2
Question 2
20 pts
What types of elements make up salt and what type of compound is it?
nonmetals only and covalent
metals only and ionic
metals and nonmetals and ionic
Answers
This is metal (Na) and nonmetal (Cl)
This makes it ionic!!!
When 1.00 mol of ethanol was mixed with 2.00 mol of acid in a 1.00 L flask, 0.86 mol of ester was formed at room temperature. What is the value of the equilibrium constant, Kc
Answers
The value of the equilibrium constant, Kc, for this reaction is approximately 0.43 L/mol.
The balanced chemical equation for the reaction between ethanol and acid to form ester is:
CH₃CH₂OH + RCOOH ⇌ CH₃COOC₂H₅ + H₂O
where R represents the organic acid group.
From the given information, the initial concentration of ethanol and acid in the flask is 1.00 mol/L and 2.00 mol/L, respectively. At equilibrium, the concentration of ester is 0.86 mol/L.
The equilibrium constant expression for the reaction is:
Kc = [CH₃COOC₂H₅][H₂O]/[CH₃CH₂OH][RCOOH]
where the square brackets represent the molar concentrations of the respective species at equilibrium.
Substituting the given values, we get:
Kc = (0.86 mol/L) / (1.00 mol/L x 2.00 mol/L) = 0.43 L/mol
Therefore, the value of the equilibrium constant, Kc, is 0.43 L/mol.
To know more about equilibrium constant click on below link :
https://brainly.com/question/31321186#
#SPJ11
Alice added sodium chloride to water and stirred the water for several minutes. Alice is most likely trying to demonstrate that ionic compounds.
Answers
Sodium chloride is an ionic compound. The chemical name of Sodium chloride is NaCl.
What are ionic compounds?Ionic compounds are made up of ions. They have charged particles. Ionic compounds when dissolved in solvents they form ions. Sodium chloride losses Na + and cl ions. Magnesium oxide will form mg2+ and O2 ions.
Ionic compounds dissolve in polar solvents. Examples are water, methanol and formamide. For ionic compounds to dissolve there will be ionic compounds will form.
Ionic bonds are not directional. There would be electrostatic or columbic attraction will be form in molecules. The bonding seen in ionic compounds is called ionic bonding. There are two types of ions seen in molecules such as positive ions and negative ions.
Therefore, Sodium chloride is an ionic compound. The chemical name of Sodium chloride is NaCl.
To learn more about ionic bonding, refer to the link:
https://brainly.com/question/11527546
#SPJ4
Answer: B
Explanation: JUST TOOK THE QUIZ
What type of stress results when two plates converge? Compression Shear Hot spot
Answers
Answer:
Compression
Explanation:
Hi! When two convergent plates collide, they should create compressive pressure, or compression.
1. How many grams of C2H2 will be produced, if 7.00g of Ca(OH)2 are also produced
in the following reaction?.
CaC2 + 2H2O -> C2H2 + Ca(OH)2
Answers
what is the name of the group that allows for the polymerization of two monosaccharides to occur? group of answer choices A. glycoside B. anhydride C. oligosaccharide D. Lactone E, hemiacetal
Answers
The correct answer is A. Glycoside. Glycosides are a type of molecule that allows for the polymerization of two monosaccharides to occur.
During the polymerization process, a glycosidic bond is formed between the two monosaccharides. This bond is created through a dehydration reaction, in which a molecule of water is removed from the two monosaccharides. The resulting molecule is called a disaccharide, which is a type of oligosaccharide.
Anhydrides, lactones, and hemiacetals are all different types of molecules that do not allow for the polymerization of monosaccharides. Anhydrides are formed from the reaction of two carboxylic acids, lactones are formed from the reaction of a carboxylic acid and an alcohol, and hemiacetals are formed from the reaction of an aldehyde and an alcohol. None of these molecules are involved in the polymerization of monosaccharides.
In summary, the name of the group that allows for the polymerization of two monosaccharides to occur is glycoside.
To know more about glycoside refer here:
https://brainly.com/question/29717563#
#SPJ11
Hydrazine (N₂H4), a rocket fuel, reacts with oxygen to form nitrogen gas and water vapor. The reaction is represented with the equation:
N₂H4(1) + O₂(g) → N₂(g) + 2H₂O(g)
How many grams of hydrazine are needed to produce 96.0g water?
Answers
Answer: To determine how many grams of hydrazine are needed to produce 96.0 grams of water, we can use the mole ratio between hydrazine and water in the chemical equation.
First, we need to convert the number of grams of water to moles. We can do this using the molar mass of water, which is 18.0 grams/mole:
96.0 g H2O / 18.0 g/mol = 5.33 moles H2O
Next, we can use the mole ratio between hydrazine and water in the chemical equation to determine the number of moles of hydrazine that would be needed to produce 5.33 moles of water:
1 mole N2H4 / 2 moles H2O = 0.5 mole N2H4
Finally, we can use the molar mass of hydrazine (32.0 grams/mole) to convert the number of moles of hydrazine to grams:
0.5 mole N2H4 * 32.0 g/mol = 16.0 grams N2H4
Therefore, 16.0 grams of hydrazine are needed to produce 96.0 grams of water.
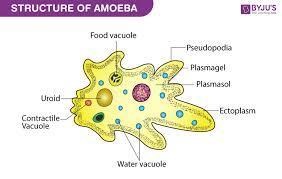
what is meant by amoeba and what is the name of its parts
Answers
Answer:
amoeba is the unicellular organism which can be seen by only microscope but not with our nacked eyes
Answer:
An amoeba often called an amoeboid, is a type of cell or unicellular organism which has the ability to alter its shape, primarily by extending and retracting pseudopods Amoebae do not form a single taxonomic group; instead, they are found in every major lineage of eukaryotic organisms. Amoeboid cells occur not only among the protozoa, but also in fungi, algae, and animals.
name of parts of ameoba
3 parts – the cytoplasm, plasma membrane and the nucleus. The cytoplasm can be differentiated into 2 layers – the outer ectoplasm and the inner endoplasm. The plasma membrane is a very thin, double-layered membrane composed of protein and lipid molecule
i hope this will help you
Ammonia gas occupies a volume of 450. mL at a pressure of 720. mm Hg. What volume will it occupy at standard pressure?
Show your work Please!!!!
Answers
V1 = 450 ml
P1 pressure equals 720 mmHg.
P2 is the standard pressure (1 atm is 760 mmHg).
V2 = the volume,
By applying Boyle's law,
P1V1 =P2V2V2 = P1V1/ P2
= (720 × 450) / 760V2
= 426 ml
426 ml is the volume at standard pressure.
The soil organic matter in Kenya has a stable carbon isotopic composition δ13C of -18 permil. Assuming that the air 13C value is -7 permil, what is the relative contribution of C3 and C4 plants to this organic matter? (please do not copy paste from previous answers from here)
Answers
Based on the given isotopic composition, the relative contribution of C3 plants is higher compared to C4 plants in the soil organic matter of Kenya.
To determine the relative contribution of C3 and C4 plants to the soil organic matter in Kenya based on their stable carbon isotopic composition, we can use the concept of isotopic discrimination.
C3 and C4 plants have different photosynthetic pathways, and they exhibit distinct carbon isotope signatures. C3 plants typically have a more negative δ13C value (around -30 permil to -22 permil), while C4 plants have a less negative δ13C value (around -16 permil to -9 permil).
In this case, the soil organic matter in Kenya has a δ13C value of -18 permil, while the air δ13C value is -7 permil. The difference between these values (-18 permil - (-7 permil)) gives us the isotopic discrimination between the atmosphere and the soil organic matter.
δ13C discrimination = δ13C organic matter - δ13C atmosphere
δ13C discrimination = -18 permil - (-7 permil)
δ13C discrimination = -11 permil
Since the δ13C discrimination is negative, it suggests that C3 plants have a dominant contribution to the soil organic matter. C4 plants, with their less negative δ13C values, are less likely to contribute significantly to the organic matter in this case.
Therefore, based on the given isotopic composition, the relative contribution of C3 plants is higher compared to C4 plants in the soil organic matter of Kenya.
Learn more about isotopic composition from the link given below.
https://brainly.com/question/10969592
#SPJ4
Explain why cheese curds form when lemon juice is added to milk. Consider the mechanism that keeps colloid particles suspended and how that mechanism could be interrupted.
Answers
The cheese curds are formed due to the coagulation of the protein by acidic lemon juice.
Why does the cheese curds form?We need to review the fundamental nature of the milk and the lemon juice. In this case, it must occur to us again that milk is made up of a protein and since it is a protein, we know that there is an optimum pH where a protein is able to function quite well. Outside that pH, the protein may not be able to function well.
Again the lemon juice is largely composed of acid since it is of the citrus family hence it contains the citric acid and this would cause the pH of the system to ow tilt towards the acid range.
Having laid these foundations, we can now see that when we do add the lemon juice to the milk the pH of the system is now outside the optimal and the protein in the milk would coagulate thus the cheese curds are formed.
Learn more about milk protein:https://brainly.com/question/21265328
#SPJ1
What mass of H2 forms when
35.25 g of Al reacts with excess
hydrochloric acid?
2AI+ 6HCI→ 2AlCl3 + 3H₂
Al: 26.98 g/mol
H₂: 2.02 g/mol
[?] g H₂

Answers
35.25/26.98 (mass reacted/molar mass of Al)
2) Find number of moles of H2 produced:
Number of mol. of Al x 3/2 (based on stoichiometry)
3) Find mass of H2 formed:
Number of mol. of H2 x molar mass of H2 = 3.9588mol = 4 mol (estimated value)
What has the lowest volume 1kg lead, 1kg of iron, 1kg of gold, or 1kg of copper
Answers
Answer:
1 kg of lead occupies the smallest amount of space of the four substances
Explanation:
The densities of lead, iron, gold, and copper are 11.34 g/cm^3, 7.87 g/cm^3, 19.32 g/cm^3, and 8.96 g/cm^3, respectively. Therefore, the volumes of 1 kg of lead, iron, gold, and copper are:
Volume of 1 kg lead = 1000 g / 11.34 g/cm^3 = 87.94 cm^3
Volume of 1 kg iron = 1000 g / 7.87 g/cm^3 = 126.98 cm^3
Volume of 1 kg gold = 1000 g / 19.32 g/cm^3 = 51.93 cm^3
Volume of 1 kg copper = 1000 g / 8.96 g/cm^3 = 111.84 cm^3
As we can see, a kilogram of lead has the lowest volume of the four substances, with a volume of 87.94 cm^3. This is because lead is the densest of the four substances, which means that it has the highest mass per unit of volume. Therefore, 1 kg of lead occupies the smallest amount of space of the four substances
which two metals would you choose to make a bi-metallic strip to maximize the displacement per unit temperature change? use the l a chart to make the choice. if the bi-metallic strip has a thickness of 2 mm and an average thermal diffusivity a of 5 105 m2 /s, approximately how long will it take to respond when the temperature suddenly changes?
Answers
It will take 0.02s to respond when the temperature suddenly changes.
Thermal diffusivity is significant because it can indicate the rate of thermal expansion of a substance. Lower heat diffusion rates are characterized by high values of thermal diffusivity. The material can therefore rapidly heat up or cool down. A higher heat diffusion rate results from a lower thermal diffusivity rating. As a result, it takes a long time for the material to heat up or cool down.
Thermal diffusivity is thus used to determine the best material to utilize based on how quickly or slowly heat dissipation is needed. It is utilized when choosing the type of material to use for heat sinks.
Here A is Thermal conductivity and a is Thermal diffusivity.
Choose metals that lie as far apart on the thermal diffusivity axis as possible, because this region maximizes the thermal displacement.
Copper and steel are probably not lead because it is too soft.
Hence, Cast Iron and Aluminium are selected.
The equation of response time is:
Response Time = (t/2)² / a
Here, t is the thickness and a is Thermal diffusivity.
Convert 2mm to m, it will be 0.002m
In the above equation, substitute 0.002m for t and 5 * 10⁻5 m²/s for a.
Response time = (0.002m/2)² / 5 * 10⁻5 m²/s
Response time = 0.02s.
Learn more about thermal diffusivity here:
https://brainly.com/question/12975861
#SPJ4
How does an emerging idea differ from scientific consensus? Which best describes emerging scientific ideas?
Answers
Emerging scientific ideas are new theories or ideas that are gaining attention in the scientific community, but have not yet been fully accepted or confirmed.
Emerging ideas refer to the new and innovative ideas or theories that have yet to gain full scientific acceptance. While a scientific consensus is a view or theory that has been universally accepted and confirmed by multiple experiments or research, an emerging scientific idea is a new and unproven theory or idea that is gaining attention in the scientific community. These emerging ideas may also be referred to as scientific hypotheses. In contrast to scientific consensus, emerging scientific ideas have not yet been subjected to rigorous testing and confirmation.
They are generally proposed to explain new observations or experimental results, which have not yet been fully understood or explained by established scientific theories. Emerging scientific ideas can have the potential to challenge the current scientific consensus. If an emerging scientific idea is found to be valid, it can ultimately lead to the establishment of a new scientific consensus. For example, the emerging scientific idea of the Higgs boson particle led to the discovery of a new field in particle physics, which is now an established scientific consensus.
for such more questions on scientific
https://brainly.com/question/29886197
#SPJ8
Which is the best example of a pure substance?
gold
air
peanuts
milk
Answers
Peanuts, or milk please leave like if right
Answer:
air
Explanation:
A substance that has a fixed chemical composition throughout is called a pure substance such as water, air, and nitrogen. A pure substance does not have to be of a single element or compound.
what is the name of Mg(MnO4)2
Answers
Answer:
Magnesium permanganate
Explanation:
Answer:
Magnesium Permanganate
Explanation:
e. Which group on the periodic table has the largest number of elements? Hint the answer is not metals or nonmetals it’s more specific
Answers
Group on the periodic table has the largest number of elements is group 3
The periodic table is a tabular array of the chemical elements organized by atomic number from the element with the lowest atomic number, hydrogen, to the element with the highest atomic number and the atomic number of an element is the number of protons in the nucleus of an atom of that element
Group 3 which comes under transition metals is the longest group consisting 32 elements and it contains lanthanide means 57 to 71 and actinide series means 89 to 103 and this comes under f block family
Know more about periodic table
https://brainly.com/question/28177183
#SPJ1
you have to prepare a ph 3.55 buffer, and you have the following 0.10m solutions available: hcooh , ch3cooh , h3po4 , hcoona , ch3coona , and nah2po4 . how many milliliters of hcooh and hcoona would you use to make approximately a liter of the buffer?
Answers
To make approximately a liter of pH 3.55 buffer, you would use 8.6 mL of 0.10 M HCOOH and 13.7 mL of 0.10 M HCOONa. We would use x mL of 0.10 M HCOOH and 0.4x mL of 0.10 M HCOONA to make approximately a liter of pH 3.55 buffer.
To prepare a pH 3.55 buffer using the available 0.10 M solutions of HCOOH (formic acid) and HCOONa (sodium formate), you can use the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
For formic acid (HCOOH), the pKa is approximately 3.75. We can rearrange the equation to find the ratio of [A-]/[HA]:
3.55 = 3.75 + log([HCOONa]/[HCOOH])
log([HCOONa]/[HCOOH]) = -0.20
[HCOONa]/[HCOOH] = 10^(-0.20) ≈ 0.63
Now, to make approximately a liter of buffer with a 0.10 M concentration, we can use the following:
0.10 L * (x + y) = 1 L
Since the ratio of [HCOONa]/[HCOOH] is 0.63, we can write:
x = 0.63y
Substitute x in the first equation:
0.10 L * (0.63y + y) = 1 L
0.73y = 10 L
y ≈ 13.7 L
Then, x ≈ 0.63 * 13.7 L ≈ 8.6 L
Learn more about pH here: https://brainly.com/question/15289714
#SPJ11