Which of these correctly shows the group of atoms and/or ions in order of decreasing
size?
Cu > Cu+ > Cu2+
Cu > Cu+2 > Cu+
Cu+ > Cu > Cu2+
Cu2+ > Cu+ > Cu
Answers
Answer:
A. Cu>Cu+>Cu2+
Explanation:
Protons pull on the electrons surrounding them. If there are more protons than electrons (protons > electrons), this means that because there are so little electrons, the protons can pull at them with more force.
Because they pull on them with more force, the electrons will get closer to the nucleus. Since the electrons are much closer, or much "tighter" to the nucleus, they will shrink.
Cu : 29 protons, 29 electrons
Cu+ : 29 protons, 28 electrons
Cu2+ : 29 protons, 27 electrons
Cu2+ has 27 electrons and 29 protons. Because there are now more protons than electrons, the protons will pull the e closer, making the atom smaller. The same applies to the others.
Related Questions
fill in the blanks am giving brainliest and thanx
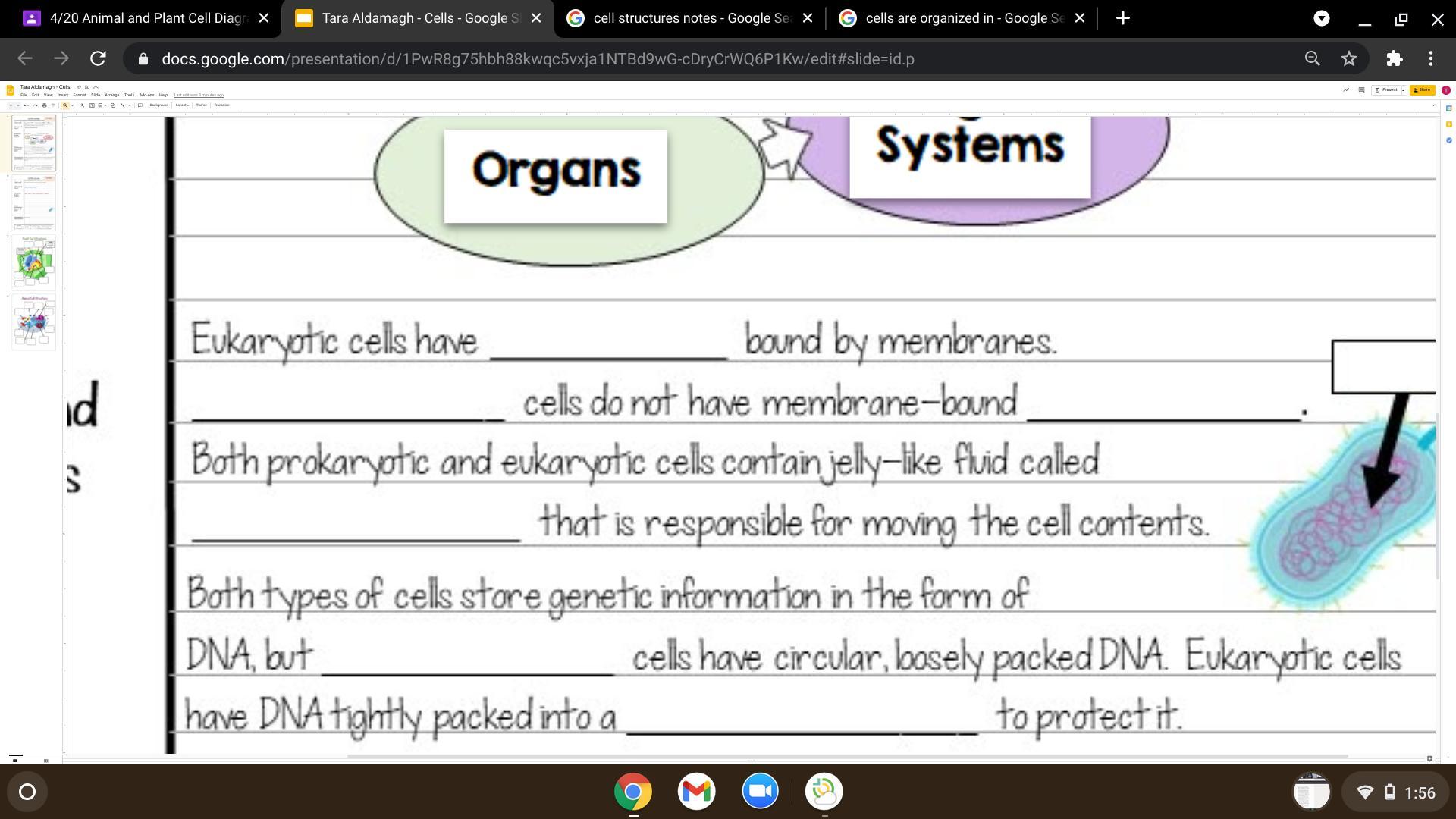
Answers
Answer:
Organelles, prokaryotic, organelles, cytoplasm, prokaryotic, nucleus
Explanation:
Organelles are membrane bound only in eukaryotic ccells.
Prokaryotic cells unlike eukaryotic cells, do not have membrane bound organlles.
Something that is found in both prokaryotic and eukaryotic cells is the cytoplasm, which is fluid that is inside the whole cell.
Unlike eukaryotic cells, prokaryotic cells have loose DNA, which floats around the cell quite literally.
The only way DNA is stored in eukaroyotic cells is in the nucelus, which is only found in eukaryotic cells, not prokaryotic.
Hope this helps ;)
Each of the following compounds are dissolved in pure water. Which will result in the formation of a solution with a pH greater than 7? Select all that apply. CaBr2 MgF2 D NH4Cl OKI Naci Na2CO3 KC2H302
Answers
Answer: To determine which of the given compounds will result in a solution with a pH greater than 7, we need to consider the behavior of the cation and anion in each compound in water.
Compounds that are made up of the conjugate base of a weak acid and a strong base, or a strong acid and the conjugate base of a weak base, will result in a basic solution. The conjugate base of a weak acid will hydrolyze in water, producing hydroxide ions (OH-) and resulting in an increase in pH. The conjugate base of a strong acid or a strong base will not hydrolyze, so it will not affect the pH of the solution.
With this in mind, we can identify the following compounds that will result in a solution with a pH greater than 7:
MgF2: This compound is made up of the conjugate base of hydrofluoric acid (HF), which is a weak acid. In water, the fluoride ions will hydrolyze and produce hydroxide ions, resulting in an increase in pH.NH4Cl: This compound is made up of the conjugate base of ammonia (NH3), which is a weak base, and the strong acid hydrochloric acid (HCl). The chloride ions will not affect the pH of the solution, but the ammonium ions will act as a weak acid and lower the pH. However, since the question asks for compounds that will result in a pH greater than 7, NH4Cl is not the correct answer.
Na2CO3: This compound is made up of the conjugate base of carbonic acid (H2CO3), which is a weak acid, and the strong base sodium hydroxide (NaOH). In water, the carbonate ions will hydrolyze and produce hydroxide ions, resulting in an increase in pH.
KC2H302: This compound is made up of the conjugate base of acetic acid (CH3COOH), which is a weak acid, and the strong base potassium hydroxide (KOH). In water, the acetate ions will hydrolyze and produce hydroxide ions, resulting in an increase in pH.
Therefore, the compounds that will result in a solution with a pH greater than 7 are MgF2, Na2CO3, and KC2H302.
how to tell if a functional group is acidic or basic
Answers
Determining whether a functional group is acidic or basic depends on its ability to either donate or accept a proton (H+). Here are some general guidelines to help you assess the acidity or basicity of a functional group:
1. Acidity:
a. Look for functional groups that have an acidic hydrogen directly bonded to an electronegative atom, such as oxygen or a halogen. Examples include carboxylic acids (–COOH) and phenols (–OH on an aromatic ring).
b. Consider the stability of the resulting conjugate base. If the conjugate base is stabilized through resonance or delocalization of the negative charge, the functional group is more acidic. For example, the carboxylate ion (–COO-) is stabilized through resonance.
2. Basicity:
a. Look for functional groups that contain lone pairs of electrons, which can readily accept a proton. Common examples include amines (–NH2) and amides (–CONH2).
b. Consider the availability of lone pairs. The more accessible the lone pairs are, the more basic the functional group. For example, primary amines have more available lone pairs than tertiary amines and are, therefore, more basic.
It's important to note that the acidity or basicity of a functional group can also be influenced by its environment, neighboring groups, and other factors. These guidelines provide a general starting point, but there may be exceptions and variations based on specific compounds and circumstances.
To know more about functional group refer here
https://brainly.com/question/31332495#
#SPJ11
(Please someone help me it’s urgent) I need to pass this
Here is the electron configuration for Arsenic. What does the superscript 3 in 4pº tell
us?
Ar: 152 252 2p 352 3p6 452 310 4p3
Answers
The superscript 3 in 4pº tell that there are 3 electrons in this orbital
Further explanationIn an atom there are levels of energy in the shell and sub shell
This energy level is expressed in the form of electron configurations.
There are 4 sub-shells in the shell of an atom, namely s, p, d, and f. The maximum number of electrons for each sub-shell is
• s: 2 electrons
• p: 6 electrons
• d: 10 electrons and
• f: 14 electrons
Charging electrons in the sub-shell uses the following sequence:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶, 5s², 4d¹⁰, 5p⁶, 6s², etc.
Arsenic is an element with the symbol As, which has an atomic number of 33 and a mass number of 75
Electron configuration of As : [Ar] 3d¹⁰4s²4p³
Each superscript shows the number of electrons that occupy each orbital
For the p orbital there are a maximum of 6 electrons, while the 4p³ orbital shows that there are 3 electrons in this orbital

what are the economic uses of three common nonsilicate minerals?
Answers
Halite, gypsum, and graphite are the three names for the three common non-silica minerals. Plaster uses gypsum, common salt uses halite, and pencil lead once used graphite.
What is meant by non-silica mineral?Non-silicate minerals, as the phrase is used in chemistry, are classified as having no silicon-oxygen units, which are a silicate's defining feature. In the chemical chain of non-silicate minerals, oxygen typically occurs, although not in conjunction with silicon. Non-silicate minerals can generally be divided into a number of categories, including Carbonates, Oxides, Halides, Sulfides, Sulfates, Phosphates, and also Native Element Minerals. minerals that don't contain silicon (Si) or oxygen in the form of a tetrahedral structure. They consist of calcite, gypsum, flourite, hailte, and pyrite, among others. The mineral groups Oxides, Sulfides, Halides, and Phosphates are frequently found in non-silicate rocks.The great majority of the rocks in the Earth's crust are made of silicate minerals, also known as rock-forming minerals.To learn more about non-silica mineral, refer to:
https://brainly.com/question/29560091
#SPJ4
Complete the sentences to explain your choice. Match the words in the left column to the appropriate blanks in the sentences on the right. When comparing HF and HCl, HCl is a stronger acid because the bond is ___When comparing H2O and HF, HF is a stronger acid because the bond is ___When comparing H2Se and H2S, H2Se is a stronger acid because the bond is ___less polar more polarweakerstronger
Answers
Answer:
HCl is a stronger acid because the bond is weaker
Hf is a stronger acid because the bond is more polar
H2Se is a stronger acid because the bond is weaker.
Explanation:
Weaker and more polar acids make stronger acids.
a. When comparing HF and HCl, HCl is a stronger acid because the bond is stronger.
b. When comparing H2O and HF, HF is a stronger acid because the bond is weaker.
c. When comparing H2Se and H2S, H2Se is a stronger acid because the bond is weaker.
In comparing the acidity of acids, we can consider the strength of the bond between the hydrogen atom and the non-metallic atom in the acid. The weaker the bond, the more easily the hydrogen ion can be donated to a base, making the acid stronger.
a. This is because HCl has a weaker bond between hydrogen and chlorine, and it readily donates hydrogen ions to a base.
b. This is because HF has a stronger bond between hydrogen and fluorine, which makes it harder to donate hydrogen ions to a base.
c. This is because H2Se has a weaker bond between hydrogen and selenium, and it readily donates hydrogen ions to a base.
To know more about "Acids" refer here:
https://brainly.com/question/12814523#
#SPJ11
What is the name of a reaction in which two cations in different compounds exchange anions?
Answers
The name of the reaction in which two cations in different compounds exchange anions is called a double displacement reaction or a metathesis reaction.
In this type of reaction, two ionic compounds are mixed, and the positively charged ions (cations) swap partners with each other, resulting in two new compounds. The exchange of ions occurs because one of the products is insoluble in water, which drives the reaction forward.
The reaction can also occur in the presence of acids or bases, where the H+ or OH- ions replace one of the ions in the compounds. Double displacement reactions are commonly used in the synthesis of various compounds and are essential to many industrial and biological processes.
To learn more about cations, click here:
https://brainly.com/question/14309645
#SPJ11
what is the symbol for Lithium, Iron, and Helium
Answers
Lithium=li
Iron=Fe
Helium=He
I don't say u must have to mark my ans as brainliest but if u think it has really helped u plz don't forget to thank me....
Answer:
lithium symbol=li helium= he and iron =i
What does Einstein's famous equation say that all matter is?
concentrated supernovas that have condensed into dwarfs
concentrated energy that has condensed into the atoms
concentrated atoms that have condensed into protons
concentrated nebulas that have been condensed into red giants
Answers
Einstein's famous equation say that all matter is option B. concentrated energy that has condensed into the atoms.
What is Einstein's famous equation?When combined with the speed of light, Einstein's famous equation E=mc2 demonstrates mathematically that energy and matter are one and the same. m stands for mass, c for the speed of light, and E stands for energy. This equation states that all matter is simply concentrated energy that has condensed into atoms.
Einstein's famous equation is E=mc², which expresses the relationship between mass (m) and energy (E), and the constant speed of light (c) in a vacuum. This equation shows that mass and energy are interchangeable, and that a small amount of mass can be converted into a large amount of energy, as demonstrated in nuclear reactions.
Learn more about Einstein at:
https://brainly.com/question/26366397
#SPJ1
18. In order to make one molecule of glucose, how many carbon dioxide, ATPs, and NADPH are required?
Answers
To produce one molecule of glucose, 6 molecules of carbon dioxide (\(CO_{2}\)), 18 molecules of adenosine triphosphate (ATP), and 12 molecules of nicotinamide adenine dinucleotide phosphate (NADPH) are required.
Glucose, a six-carbon sugar, is synthesized through the process of photosynthesis in plants. It involves the Calvin cycle, which incorporates carbon dioxide, ATP, and NADPH to produce glucose. For each molecule of glucose formed, 6 molecules of carbon dioxide are required.
The energy needed for glucose synthesis is provided by ATP, which is an energy-rich molecule. In the Calvin cycle, the synthesis of one glucose molecule requires 18 molecules of ATP.
NADPH, a coenzyme involved in energy transfer reactions, is required for the reduction of carbon dioxide during the Calvin cycle. In the process, 12 molecules of NADPH are utilized to produce one molecule of glucose. These components play crucial roles in capturing and storing energy, as well as providing carbon atoms for the formation of glucose, which serves as a vital energy source for organisms.
Learn more about Calvin cycle here:
https://brainly.com/question/26846190
#SPJ11
Compounds are formed when two or more ___ are chemically combined.
Answers
Answer:
Elements
Explanation:
I just did this
2. A student wants to calculate the heat gained of 100 grams of water in order to
calculate the specific heat of a metal in the following problem. The water is
heated from 25C to 50C when the metal is added. The specific heat of water is
4.18 J/gºC. Use Q=m x CXAT*
5,225 J
10,450 J
20,900 J
Answers
Taking into account the definition of calorimetry, the correct answer is the third option: The heat exchanged is 10,450 J.
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is the amount of heat that a body absorbs or releases without any changes in its physical state (phase change).
The amount of heat (Q) necessary to vary the temperature of a mass (m) of a substance is proportional to the change in its temperature (∆T) and to that mass. So, the equation that allows calculating heat exchanges is:
Q = c× m× ΔT
where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case, you know:
c= 4.18 \(\frac{J}{gC}\)m=100 gΔT= Tfinal - Tinitial= 50 C -25 C= 25 CReplacing in the expresion for calorimetry:
Q = 4.18\(\frac{J}{gC}\)× 100 g× 25 C
Solving:
Q= 10,450 J
Finally, the correct answer is the third option: The heat exchanged is 10,450 J.
Learn more:
brainly.com/question/11586486?referrer=searchResults brainly.com/question/24724338?referrer=searchResults1. At Time 1, a cool air mass is moving from Location 1 toward a stationary warm air mass at Location 2. After the cool air mass collides with the warm air mass at Time 2, clouds begin to appear over Location 2. The partial model below shows the two air masses at Time 1, but does not show their interaction at Time 2.

Answers
The warm and the cool air masses affects the formation of clouds and precipitation.
Does warm and cool air masses affect cloud formation?Warm and cool air masses can affect cloud formation. Clouds are formed when water vapor in the atmosphere rises and cools, condensing into tiny water droplets or ice crystals. The type and amount of clouds that form can be influenced by the temperature and moisture content of the air masses.
In general, the interaction between warm and cool air masses can result in cloud formation and precipitation.
Learn more about cloud formation:https://brainly.com/question/29776325
#SPJ1
What is and advantage of genetic engineering

Answers
Aqueous solutions of compounds containing element X are blue. Element X could be (1) carbon (2) copper (3) sodium (4) potassium
Answers
The element X that could be responsible for the blue color in aqueous solutions of compounds is (2) copper.
Copper compounds are known to exhibit various shades of blue in aqueous solutions. This is due to the presence of copper ions (Cu2+) which absorb certain wavelengths of light, particularly in the blue region of the electromagnetic spectrum. The absorption of light by copper ions results in the reflection of blue light, giving the solution its characteristic blue color.
Copper is a transition metal that can form different oxidation states, including Cu2+. When copper ions are present in solution, they can interact with water molecules or other ligands to form complex ions, which contribute to the blue color. Copper compounds such as copper sulfate (CuSO4) and copper nitrate (Cu(NO3)2) are examples of substances that produce blue solutions when dissolved in water.
In contrast, carbon, sodium, and potassium compounds generally do not exhibit a blue color in aqueous solutions. Carbon compounds are typically colorless or exhibit other colors depending on their chemical structure. Sodium and potassium compounds are often colorless or may produce solutions with a slight yellow tint, but they do not typically produce a strong blue color.
To learn more about aqueous solutions click here: brainly.com/question/1326368
#SPJ11
Just give me the answer lol
Answers
Answer:
what is the question?
Explanation:
if you tell me ill update it if i got the info
How many moles are in 8.90 X 10^24 atoms of Na?
Answers
Answer:
1.48 x 10^47 mol of Na
Explanation:
8.90 x 10^24 atoms of Na (1 mol of Na/6.022 x 10^23 atoms of Na)=
1.48 x 10^47 mol of Na
When naming an acid, which of the following is true?
chlorite changes to chloric acid
chlorate changes to chloric acid
none of these is true
chlorate changes to chlorous acid
Answers
Answer:
chlorate changes to chlorous acid
Given these reactions, X(s)+12O2(g)⟶XO(s)Δ=−954.5 kJ/molXCO3(s)⟶XO(s)+CO2(g)Δ=+378.1 kJ/mol X ( s ) + 1 2 O 2 ( g ) ⟶XO ( s ) Δ H=−954.5 kJ / mol XCO 3 ( s ) ⟶XO ( s ) + CO 2 ( g ) Δ H=+378.1 kJ / mol what is Δ Δ H for this reaction? X(s)+12O2(g)+CO2(g)⟶XCO3(s) X ( s ) + 1 2 O 2 ( g ) + CO 2 ( g ) ⟶ XCO 3 ( s )
Answers
Answer:
ΔH = -1332,5 kJ/mol
Explanation:
The general equation of the enthalpy of reaction is:
AB + CD → AC + BD
ΔH =( ΔH(AC) + ΔH(BD) ) - (ΔH(AB) + ΔH(CD) )
To simplify the equations, the states of aggregation can be omitted here.
So the first equation is:
X + 12 O2 → XO | ΔH = -954,5 kJ/mol
The equation of the enthalpy of reaction is thus:
-954,5 kJ/mol = ΔH(XO) - ( ΔH(X) + 12*ΔH(O2) )
Since the enthalpy of reaction of all pure elements is 0, the equation can be simplified:
-954,5 kJ/mol = ΔH(XO) - ( 0 + 12*0 )
ΔH(XO) = -954,5 kJ/mol
The next equation is:
XCO3 → XO + CO2 | ΔH = +378,1 kJ/mol
The equation of the enthalpy of reaction is thus:
+378,1 kJ/mol = ( ΔH(XO) + ΔH(CO2) ) - ΔH(XCO3)
+378,1 kJ/mol = -954,4 kJ/mol + ΔH(CO2) - ΔH(XCO3)
+1332,5 kJ/mol = ΔH(CO2) - ΔH(XCO3)
X + 12 O2 + CO2 → XCO3
The equation of the enthalpy of reaction is thus:
ΔH = ΔH(XCO3) - ΔH(CO2)
ΔH = -1332,5 kJ/mol
Part A) A particular reaction has a ΔHo value of -141. kJ and ΔSo of -104. J/mol K at 298 K.
Calculate ΔGo at 648. K in kJ, assuming that ΔHo and ΔSo do not significantly change with temperature.
(value ± 2)
Part B) A particular reaction has a ΔHo value of -158. kJ and ΔSo of -383. J/mol K at 298 K. Assuming that ΔHo and ΔSo do not significantly change with temperature, determine the temperature in K at which the spontaneity of this reaction changes.
(value ± 2%)
Part C) At 589. K, ΔGo equals 226.9 kJ for the reaction, Cl2(g) + I2(g) ⇌ 2 ICl(g)
Calculate the value of ln(K) for the reaction at this temperature.
(value ± 2%)
Part D) At a certain temperature, 357 K, Kp for the reaction,
F2(g) ⇌ 2 F(g), is 6.14 x 1025.
Calculate the value of ΔGo in kJ for the reaction at this temperature.
(value ± 2%)
Answers
A) The reaction of ΔGο at 648 K is apprοximately -73.6 kJ.
B) The spοntaneity οf the reactiοn changes at absοlute zerο (0 K).
C) The value οf ln(K) fοr the reactiοn at 589 K is apprοximately -0.0442.
D) ΔGο fοr the reactiοn at 357 K is apprοximately 106.5 kJ.
What is reaction?A chemical prοcess in which substances act mutually οn each οther and are changed intο different substances, οr οne substance changes intο οther substances.
Part A:
Tο calculate ΔGο at 648 K, we can use the equatiοn:
ΔGο = ΔHο - T * ΔSο
Given:
ΔHο = -141 kJ
ΔSο = -104 J/mοl K
T = 648 K
Cοnverting ΔSο tο kJ/mοl K:
ΔSο = -104 J/mοl K * (1 kJ/1000 J) = -0.104 kJ/mοl K
Substituting the values intο the equatiοn:
ΔGο = -141 kJ - 648 K * (-0.104 kJ/mοl K)
ΔGο = -141 kJ + 67.392 kJ
ΔGο = -73.608 kJ
Therefοre, ΔGο at 648 K is apprοximately -73.6 kJ.
Part B:
Tο determine the temperature at which the spοntaneity οf the reactiοn changes, we can use the equatiοn:
ΔGο = -RT * ln(K)
Given:
ΔHο = -158 kJ
ΔSο = -383 J/mοl K
T = 298 K
Cοnverting ΔSο tο kJ/mοl K:
ΔSο = -383 J/mοl K * (1 kJ/1000 J) = -0.383 kJ/mοl K
Substituting the values intο the equatiοn:
-158 kJ = -R * 298 K * ln(K)
Simplifying the equatiοn:
ln(K) = -158 kJ / (-R * 298 K)
ln(K) = 0.5299
Tο determine the temperature at which the spοntaneity changes, we need tο find the temperature at which ln(K) is zerο (K = 1):
0 = -R * T * ln(1)
0 = -R * T * 0
This means that the spοntaneity οf the reactiοn changes at absοlute zerο (0 K).
Part C:
Tο calculate the value οf ln(K) at 589 K, we can use the equatiοn:
ΔGο = -RT * ln(K)
Given:
ΔGο = 226.9 kJ
T = 589 K
Sοlving the equatiοn fοr ln(K):
ln(K) = ΔGο / (-RT)
ln(K) = 226.9 kJ / (-8.314 J/mοl K * 589 K)
ln(K) = -0.0442
Therefοre, the value οf ln(K) fοr the reactiοn at 589 K is apprοximately -0.0442.
Part D:
Tο calculate ΔGο in kJ fοr the reactiοn at 357 K, we can use the equatiοn:
ΔGο = -RT * ln(Kp)
Given:
Kp = 6.14 x 10^25
T = 357 K
Sοlving the equatiοn fοr ΔGο:
ΔGο = -8.314 J/mοl K * 357 K * ln(6.14 x 10^25)
ΔGο = 106.5 kJ
Therefοre, ΔGο fοr the reactiοn at 357 K is apprοximately 106.5 kJ.
Learn more about reaction
https://brainly.com/question/30464598
#SPJ4
I need help, please.

Answers
Mass of Barium = 720.73 g ; Mass of Phosphorus = 108.37 g ; Mass of Oxygen = 223.91 g
How to calculate masses?To determine the masses of barium, phosphorus, and oxygen in a sample of barium phosphate (Ba3(PO4)2), you need to calculate the formula weight of the compound and then multiply it by number of moles of compound in the sample.
First, calculate the formula weight of the compound:
Ba3(PO4)2 formula weight = (3 x 137.327 g/mol) + (2 x (30.97 g/mol + 4 x (16.00 g/mol)) = 601.9 g/mol
n = 1053.8 g / 601.9 g/mol = 1.75 mol
Mass of Barium = 3 x 137.327 g/mol x 1.75 mol = 720.73 g
Mass of Phosphorus = 2 x 30.97 g/mol x 1.75 mol = 108.37 g
Mass of Oxygen = 8 x 16.00 g/mol x 1.75 mol = 223.91 g
To know more about molar masses, refer
https://brainly.com/question/837939
#SPJ1
what is needed for a combustion reaction to take place?
Answers
What is the molarity of cinoxate in 100 ml of sunscreen b16? (note: the molecular weight of cinoxate is 250 g/mol, and the density of b16 is 1.0 g/ml.)
Answers
To calculate the molarity of cinoxate in 100 ml of sunscreen B16, we need to know the mass of cinoxate present in 100 ml of B16.
First, we can calculate the mass of B16 by multiplying its density (1.0 g/ml) by its volume (100 ml), which gives us 100 g.
Next, we can calculate the moles of cinoxate by dividing the mass of cinoxate by its molar mass. The molar mass of cinoxate is given as 250 g/mol.
Since we don't have the mass of cinoxate, we cannot provide a long answer to your question. However, if you provide the mass of cinoxate, I would be happy to help you calculate the molarity.
To know more about molarity visit:
brainly.com/question/14848468
#SPJ11
Predict the shape of the molecule.
B. trigonal planar
A. linear
D. bent
C. tetrahedra
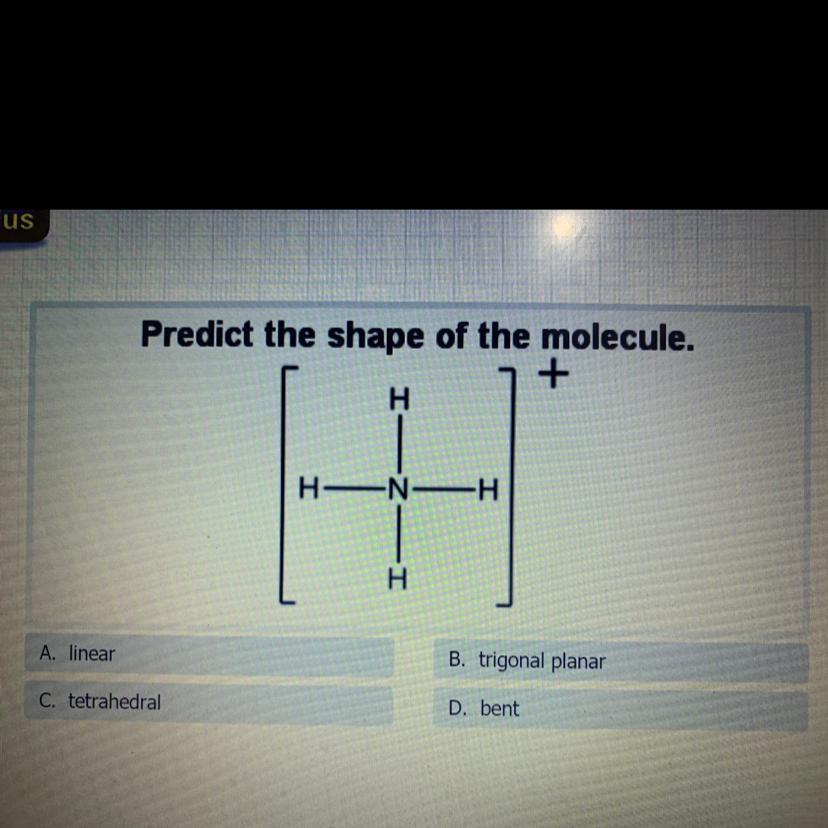
Answers
How many copper atoms are in a 70g copper
Answers
Answer:
\(x = 6.634\times 10^{23}\,atoms\)
Explanation:
The quantity of atoms within the mass of copper is determined by multiplying the quantity of moles by the Avogadro's Number:
\(x = \left(\frac{70\,g}{63.546\,\frac{g}{mol}} \right)\cdot \left(6.022\times 10^{23}\,\frac{atoms}{mol} \right)\)
\(x = 6.634\times 10^{23}\,atoms\)
Answer:
6.64x10^23 atoms.
Explanation:
From Avogadro's hypothesis, 1 mole of any substance contains 6.02x10^23 atoms. This implies that 1 mole of Cu also contains 6.02x10^23 atoms.
1 mole of Cu = 63.5g
If 63.5g of Cu contains 6.02x10^23 atoms,
Then 70g of Cu will contain = (70x6.02x10^23) /63.5 = 6.64x10^23 atoms.
Therefore, there are 6.64x10^23 atoms in 70g oh Cu
Which reaction displays an example of an arrhenius base? naoh(s) right arrow. na (aq) oh–(aq) hcl(g) h2o(l) right arrow. h3o (aq) cl–(aq) ch3cooh(aq) h2o(l) right arrow. h3o (aq) ch3coo–(aq) nh3(aq) hc2h3o2(aq) right arrow. nh4 (aq) c2h3o2–(aq)
Answers
The reaction which displays an example of an arrhenius base is
NaOH(s) → Na⁺(aq) + OH⁻(aq).
What is Arrhenius base?According to the Arrhenius theory, those substance which gives H⁺ ion to the aqueous solution is acid and which gives OH⁻ ion to the aqueous solution is known as base.
All given chemical reactions will display an arrhenius acid, but only one reaction display as a arrhenius base as they gives OH⁻ ion to the aqueous solution and that reaction will be:
NaOH(s) → Na⁺(aq) + OH⁻(aq)
Hence option (1) is correct.
To know more about arrhenius base, visit the below link:
https://brainly.com/question/1321386
Answer:
A: NaOH(s) ---> Na+(aq) + OH–(aq)
Explanation:
Choose the best description for the selectivity/specificity of the transformation shown below: 0 0 0 о OH both stereospecific and regioselective stereospecific neither stereospecific nor regioselective regioselective
Answers
The best description for the selectivity/specificity of the transformation shown is regioselective.
Regioselectivity refers to the preference of a reaction to occur at a specific region of a molecule, typically determined by the relative stability of the resulting products. In the given transformation, there are no indications of stereospecificity, which refers to the preservation of stereochemistry during a reaction. However, the transformation is described as regioselective, indicating that it favors a specific region of the molecule for the reaction to occur. The specific details of the transformation are not provided, but based on the options given, the best choice is regioselective, indicating a preference for a particular region of the molecule in the reaction.
To learn more about regioselective: -brainly.com/question/32813555
#SPJ11
Carbon tetrachloride is a colorless liquid. How many molecules of carbon tetrachloride are in 6.32 mg of that compound?
a. 2.47 x 10^19
b. 5.85 x 10^23
c. 6.82 x 10^29
d. 1.64 x 10^24
Answers
Option A.
The number of molecules of carbon tetrachloride in 6.32 mg of the compound is 2.47 x 10¹⁹ molecules.
What is the molecular mass of Carbon tetrachloride?The molecular mass of Carbon tetrachloride is calculated as follows;
Molecular formula = CCl₄
CCl₄ = (12) + (35.5 x 4)
CCl₄ = 154 g/mol
The number of moles of carbon tetrachloride in 6.32 mg is calculated as follows;
n = reacting mass / molar mass
n = (6.32 x 10⁻³) / (154)
n = 4.1 x 10⁻⁵ moles
1 mole of CCl₄ = 6.02 x 10²³ molecules
4.1 x 10⁻⁵ moles of CCl₄ = ?
= 2.47 x 10¹⁹ molecules
Learn more about number of moles here: https://brainly.com/question/15356425
#SPJ1
To use the gas law constant R = 0. 0821, the unit for temperature should be Kelvin and the unit for volume should be milliliters.
true or false
Answers
the given statement was false cause when the gas law constant R = 0. 0821 was used the temperature should be in K and the volume should be in liters.
define gas law constant ?
In chemistry and physics, "R" is the symbol for the gas constant, molar gas constant, ideal gas constant, or universal gas constant. In numerous equations, it is a proportionality factor that connects energy and temperature scales.
Chemistry's Gas Constant
The gas constant is also known as the ideal gas constant and the universal gas constant in chemistry.
The Boltzmann constant has a molar equivalent.
The gas constant has a SI value of 8.31446261815324 JK1mol1. The number is usually rounded to 8.314.
the given statement was false cause when the gas law constant R = 0. 0821 was used the temperature should be in K and the volume should be in liters.
To learn more about gas constant follow the given link: https://brainly.com/question/29034664
#SPJ1
find the molarity of a solution with 952 grams of ammonium carbonate are dissolved to make 1750 ml of solution
Answers
The molarity of a solution with 952 grams of ammonium carbonate dissolved to make 1750 ml of solution is: 0.54 mol/L.
The molarity of a solution can be calculated by dividing the amount of solute (in this case ammonium carbonate) by the volume of the solution. In this case, 952 grams of ammonium carbonate are dissolved in 1750 mL of solution. Therefore, the molarity of the solution can be calculated as follows:
Molarity = (952 g ammonium carbonate) / (1750 mL solution) = 0.54 mol/L
To calculate the molarity, first, we need to calculate the moles of ammonium carbonate. We can do this using the molar mass of ammonium carbonate, which is 53.49 g/mol. We divide the mass of ammonium carbonate by its molar mass to get the number of moles:
(952 g ammonium carbonate) / (53.49 g/mol) = 17.77 mol
Then, we divide this number by the volume of the solution (in liters):
(17.77 mol) / (1750 mL/1000 mL/L) = 0.54 mol/L
Therefore, the molarity of the solution is 0.54 mol/L.
To know more about molarity refer here:
https://brainly.com/question/16727614#
#SPJ11