Answers
Answer:
is there an image
Explanation:
Related Questions
0/1 point
Click on the chemical compound that is a product in
this balanced chemical equation.
N+3H, → ẢNH,
Answers
NH3 is the chemical compound produced as a product in the given balanced chemical equation.
What is NH3 defined in chemistry?With the formula NH3, ammonia is a nitrogen and hydrogen inorganic chemical. A Lewis base is urea. Ammonia is a colourless, extremely unpleasant gas that has a strong, suffocating stink when it is present at room temperature. It is hygroscopic and known as anhydrous ammonia in its pure state (readily absorbs moisture). Ammonia is corrosive and has alkaline qualities.
Do NH3 and H2O have such a dipole moment?Water molecules' shared dipoles and development of hydrogen bonds with one another account for this. As a consequence, the molecules of water and ammonia are both made up of dipoles that exert significant electrostatic forces on one another.
Learn more about chemical equation here:
brainly.com/question/14072552
#SPJ1
true or false is NO3- the only form of nitrogen used by higher plants.
Answers
Answer:
True
Explanation:
Nitrate is in most plants
what does it mean to count atoms
Answers
When you count atoms, remember to multiply the coefficient number and the subscript number to find the number of atoms of that element. Examples: ▪ 2H2O. Number of atoms Number of molecules = 2. H-4.
Calculate the cell potential (Ecell) at 25oC (298 K) for the following reaction if the Cu2+ ion concentration is 0.064 M and the Fe2+ ion concentration is 0.645 M.
Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
Half-reaction Standard Reduction Potential (V)
Fe2+(aq) + 2e−→ Fe(s) −0.440
Cu2+(aq) + 2e−→ Cu(s) +0.337
R = 8.31 V/mol·K
F = 96500 C/mol
Answers
The cell potential (Ecell) at 25°C (298 K) for the given reaction is 1.065 V which means that the reaction is spontaneous because the calculated Ecell is positive
The cell potential (Ecell) for the given reaction can be calculated using the Nernst equation:
Ecell = E°cell - (RT ÷ nF) × ln(Q)
where E°cell = standard cell potential, R = gas constant, T = temperature in Kelvin, n = number of electrons transferred in the reaction, F = Faraday constant, and Q is the reaction quotient.
Since two electrons are transported in the half-reactions, n in this instance equals 2. With the help of the species concentrations, it is possible to determine the reaction quotient Q:
Q = [Fe2+] ÷ [Cu2+]
Q = 0.645 ÷ 0.064
Q = 10.078
Now, we can calculate the Ecell:
Ecell = E°cell - (RT ÷ nF) × ln(Q)
Ecell = (0.337 - (-0.440)) - (8.31 × 298 ÷ (2 × 96500)) × ln(10.078)
Ecell = 1.065 V
To learn more about cell follow the link:
https://brainly.com/question/1313684
#SPJ1
How long did it take a bike to travel 20 miles at 12 mph
Answers
Answer: 4 miles
Explanation:
Riding the bike 12 mph (miles per hour) for one hour, we would ride the bike 12 miles. Since 20 minutes is 1/3 of a hour, divide 12 by 3 to find out how far the biker traveled in 1/3 of the time: 4 miles Hope this helps
Hello!
\(\large\boxed{\frac{5}{3}\text{ hours} \text{ or }100 \text{ min}}\)
Use the equation d = s · t where:
s = speed
t = time
d = distance
Plug in the given distance and speed:
20 = 12 ·t
Divide both side by 12 to isolate for t:
20/12 = t
Simplify:
5 / 3 = t
Therefore, it took 5/3 hours to bike. We can convert this to minutes if necessary:
5/3 · 60 min (in one hour) = (60 · 5) / 3 = 100 min.
Look at the diagram of a fuel cell below. A fuel cell with 2 vertical objects labeled A and B connected by an electrical wire through a circle with a M in it. There is an area between the two vertical objects labeled A, and substances flowing to, along, and away from the vertical objects and to the left and right. Which statement describes how electrons move if oxidation occurs on the left side of the cell and reduction occurs on the right side? Electrons move from left to right through Electrons move from right to left through A. Electrons move from left to right through M. Electrons move from right to left through M.
Answers
The electrons move from left to right through the circle labeled "M" to reach the cathode, where reduction takes place.
If oxidation occurs on the left side of the fuel cell and reduction occurs on the right side, the movement of electrons can be described as follows: Electrons move from left to right through the circle labeled "M."
In a fuel cell, the process of oxidation takes place at the anode (labeled A) where the fuel is oxidized, releasing electrons. These electrons then flow through the external electrical circuit, represented by the wire connecting objects A and B. The electrons reach the cathode (also labeled A) on the right side of the cell, where reduction occurs.The circle labeled "M" represents the membrane or electrolyte in the fuel cell. This membrane allows the transport of ions but blocks the movement of electrons. As a result, electrons cannot flow directly through the electrolyte but must travel through the external circuit.
This movement of electrons through the external circuit is what generates an electric current that can be used to power electrical devices or systems.
for such more questions on reduction
https://brainly.com/question/21851295
#SPJ8
What happens if more solute is added to a saturated solution?
Answers
Answer:
A saturated solution is a mixture in which the maximum amount of a given solute has been dissolved into the solvent. ... At this point adding more solute will not change the concentration of the solution; adding more solute will simply result in more solid at the bottom of the solution.
Answer:
will not change
Explanation:
A saturated solution is a mixture in which the maximum amount of a given solute has been dissolved into the solvent. ... At this point adding more solute will not change the concentration of the solution; adding more solute will simply result in more solid at the bottom of the solution.
What are the mass numbers for the two nitrogen isotopes?
N-14
N-15
N-25
N-13
Answers
The 2 isotopes of Nitrogen are Nitrogen-14 and Nitrogen-15 .
What are isotopes?Isotopes are atoms with the same number of protons and different number of neutrons. Even though they have nearly identical chemical properties, they differ in mass which affects their physical properties.
As you know, the atomic number of nitrogen is = 7.
Isotopes are forms of elements that have the same atomic number (Z) but different mass numbers because they have different numbers of neutrons (A).
Now,
Atomic number = number of protons
Isotopes have the same atomic number.
Atomic number for both isotopes = 7. However, the mass numbers of the two isotopes are different.
In other words, the mass number = the number of (protons + electrons).
Therefore, nitrogen-14 has a mass number of 14 and nitrogen-15 has a mass number of 15.
To know more about isotopes refer to:
https://brainly.com/question/14220416
#SPJ1
strong lemonade in a pewter pitcher can result in
Answers
Strong lemonade in a pewter pitcher can result in chemical contamination.
The type of Utensils and equipment used to cook or store food and beverages can cause a detrimental effect to the body.
A pewter pitcher is a kitchen utensil for storing primarily water; it is made of lead, tin, copper, and occasionally silver alloy.When fruit juices like lemon, orange or tomato are stored in pewters.They cause a chemical reaction to occur from the acid contained in fruit juices with the metal on the the juice becomes contaminated chemically when pewter is consumed, causing parts or particles of the metal to dissolve in the juice and harming consumption.Learn more about chemical reaction at:
brainly.com/question/29039149
#SPJ4
Complete Question
Storing Lemonade in a pewter pitcher can result in Chemical contamination.
The options were omitted an they are
A. Physical contamination
B. Chemical contamination
C. Cross-contact
Elements in the same period of the periodic table exhibit similar physical and chemical properties.
Answers
Answer:
same valency electrons
Explanation:
example g 1 elements
In a particular redox reaction, NO is oxidized to NO−3
and Cu2+ is reduced to Cu+.
Complete and balance the equation for this reaction in acidic solution. Phases are optional.
balanced redox reaction:
NO + Cu^{2+} -> NO_{3}^{-} + Cu^{+}
Answers
The balanced redox equation for the given reaction in acidic solution is:
\(3NO + 3Cu^2+ + 2e^- - > 3NO_3^- + 3Cu^+.\)
To balance the redox reaction:
\(NO + Cu^2+ - > NO_3^- + Cu^+\)
First, let's assign oxidation states to each element/ion in the equation:
Oxidation state of N in NO: +2
Oxidation state of N in \(NO_3^-: +5\)
Oxidation state of Cu in \(Cu^2+: +2\)
Oxidation state of Cu in \(Cu^+: +1\)
From the given oxidation states, we can see that N is being oxidized from +2 to +5, and Cu is being reduced from +2 to +1. We need to balance both the charge and the number of atoms on each side of the equation.
Balancing the nitrogen:
We need three NO molecules on the reactant side to balance the nitrogen atoms on the product side. Thus, the equation becomes:
\(3NO + Cu^2+ - > NO_3^- + Cu^+\)
Balancing the charge:
The charge on the reactant side is 0 (since NO is neutral), while on the product side, we have 1- charge from NO_3^- and 1+ charge from Cu^+. To balance the charges, we need two electrons on the reactant side.
Final balanced equation:
\(3NO + 3Cu^2+ + 2e^- - > 3NO_3^- + 3Cu^+\)
In acidic solution, we need to balance the hydrogen ions \((H^+)\). In this case, there are no hydrogen ions on either side of the equation, so no additional steps are needed.
For more such information on: redox equation
https://brainly.com/question/27907895
#SPJ8
Elements in which family would require the least amount of ionization energy (the
amount of energy used to take away an electron)?
transition metals
Alkali metals
Alkaline earth metals
Answers
Alkali metals require least amount of ionization energy.
Peaky Blinder fan? haha
One mole of oxygen gas is at a temperature of 30°C. If the gas is heated at constant volume until the pressure triples, what is the final temperature?
A. 60°C
B. 50°C
C. 363 K
D. 423 K
tysm! :)
Answers
Considering the Gay-Lussac's law, the final temperature when the gas is heated at constant volume until the pressure triples is 909 K or 636° C.
Gay-Lussac's lawGay-Lussac's law establishes the relationship between the temperature and pressure of a gas when the volume is constant. This law says that the pressure of a gas is directly proportional to its temperature: if the temperature increases, the pressure will increase, while if the temperature decreases, the pressure will decrease.
Mathematically, Gay-Lussac's law states that the ratio between pressure and temperature always had the same value:
P÷ T= k
where
P is the pressure.T is the temperature.k is a constant.Analyzing an initial state 1 and a final state 2, it is fulfilled:
P₁÷ T₁= P₂÷ T₂
Final temperatureIn this case, you know that the gas is heated at constant volume until the pressure triples. This is, P₂=3×P₁
Replacing in Gay-Lussac's law:
P₁÷ T₁= (3×P₁)÷ T₂
T₂× (P₁÷ T₁)=(3×P₁)
T₂= (3×P₁)÷ (P₁÷ T₁)
T₂= 3×T₁
If one mole of oxygen gas is at a temperature of 30°C of 303 K (being 0 C= 273 K), then:
T₂= 3×303 K
T₂= 909 K = 636° C
Finally, the final temperature is 909 K or 636° C.
Learn more about Gay-Lussac's law:
brainly.com/question/30085244
brainly.com/question/2683502
#SPJ1
How many moles of oxygen are produced when 494 grams of water decomposes?
2H2O (l) --> 2H2 (g) + O2 (g)
Answers
Two moles or 36 g of water on decomposition gives 32 grams of oxygen gas. Then 494 g of water will give 439.1 g of oxygen.
What is decomposition reactions?The decomposition is a type of reaction in which a single compound breaks into its constituent elements or molecules. Water molecules are formed by the combination of hydrogen and oxygen atoms.
Decomposition of two moles of water decompose to give 2 moles of hydrogen gas and one mole of oxygen gas.
molar mass of water = 18 g/mol
molecular mass of oxygen = 32 g/mol
Two moles or 36 g of water gives one mole or 32 g of oxygen gas. Then mass of oxygen produced by 494 g of water is :
(494 ×32)/36 g = 439 g
Therefore, 439 g of oxygen gas is formed by the decomposition of 494 g of water.
Find more on decomposition reactions :
https://brainly.com/question/30691871
#SPJ9
equation for the oxidation of octane.
Answers
Answer:
I think its 2 C8H18 + 25 O2 = 18 H2O + 16 CO2
Im not sure!
Explanation:
6. Convert the following measurements to the indicated units,
a. 238.4 mm to m
b. 0.00355 L to mL
c. 48.6 s to h
d. 6.50 oz to mL
Answers
Answer:
C
Explanation:
hahhahahabhahahahahahhahaha
PLEASE HELP!!!
A 84.1 g sample of phosphorus reacts with 85.0 g of oxygen gas according to the following chemical equation.
4 P(s) + 5 O2(g) → 2 P₂O5
a. Find the limiting reactant.
b. How many grams of P₂O5 are produced in theory?
c. If only 123 g of P2O5 are produced, what is the percentage yield?
Answers
Answer:
(a) oxygen
(b) 154g (to 3sf)
(c) 79.9% (to 3sf)
Explanation:
mass (g) = moles × Mr/Ar
note: eqn means chemical equation
(a)
moles of P = 84.1 ÷ 30.973 = 2.7152 moles
moles of O2 = 85÷2(16) = 2.65625 moles
Assuming all the moles of P is used up,
moles of O2 / moles of phosphorus = 5/4 (according to balanced chemical eqn)
moles of O2 required = 5/4 × 2.7152moles = 3.394 moles (more than supplied which is 2.65625moles)
therefore there is insufficient moles of O2 and the limiting reactant is oxygen.
(b)
moles of P2O5 produced
= 2/5 (according to eqn) × 2.7152
= 1.08608moles
mass of P2O5 produced
= 1.08608 × [ 2(30.973) + 5(16) ]
= 154.164g
= approx. 154g to 3 sig. fig.
(c)
% yield = actual/theoretical yield × 100%
= 123/154 × 100%
= 79.870%
= approx. 79.9% (to 3sf)
Which statement could be used as a testable hypothesis for a scientificexperiment?A. If you put a cricket in water, it will be too scared to chirp.B. Does humidity affect how rapidly crickets chirp?C. Crickets are more annoying at night than during the day.OD. If the temperature decreases, then crickets will chirp less.
Answers
Answer
D. If the temperature decreases, then crickets will chirp less.
Explanation
A hypothesis is a testable guess about the relationship between two or more variables. As the temperature rises, it becomes easier to reach a certain activation energy, thereby allowing chemical reactions, such as the ones that allow a cricket to chirp, to occur more rapidly. Conversely, as the temperature falls, the reaction rates slow, causing the chirping to diminish along with it. Therefore the above statem
consider the combustion of carbon monoxide in oxygen gas.2CO(g)+O2(g)=2CO2(g).In this reaction 10.8 moles of carbon dioxide was produced. Calculate the number of moles of carbon monoxide used in this reaction to produce such number of moles of carbon dioxide.
Answers
In this reaction 10.8 moles of carbon dioxide was produced. 10.8moles of carbon monoxide used in this reaction to produce such number of moles of carbon dioxide.
In chemistry, a mole, usually spelt mol, is a common scientific measurement unit for significant amounts of very small objects like molecules, atoms, or other predetermined particles. The mole designates 6.02214076 1023 units, which is a very large number.
For the Worldwide System of Units (SI), the mole is defined as this number as of May 20, 2019, according to the General Convention on Measurements and Weights. The total amount of atoms discovered through experimentation to be present in 12 grammes of carbon-12 was originally used to define the mole.
2CO(g)+O\(_2\)(g)=2CO\(_2\)(g)
moles of carbon dioxide= 10.8 moles
According to stoichiometry
moles of carbon monoxide= (2/2)× 10.8 =10.8moles.
To know more about mole, here:
brainly.com/question/31597231
#SPJ1
Disadvantage and advantage of iodine ?
- umoren sŭm..
Answers
THE ADVANTAGE OF IODIN
IODINE CAN LEAD TO GOITER AND OTHER THYROID PROBLEMS.THE DISADVANTAGE OF IODIN
IODIN MAY LEAD TO THYROIDITIS AND THYROID PAPILLARY CANCERflourine is more reactive than chlorine . why ? with short reason.
Answers
Answer:
Electronegativity is probably the biggest thing that plays into reactivity. Therefore, since fluorine has a higher electronegativity than chlorine, fluorine is more reactive.
Explanation:
I got it right
Write the objectives for the topic "Synthetic Fibre & Plastics"
Answers
Answer:
1. To understand the differences between natural and synthetic fibers and their properties.
2. To learn about the manufacturing process of synthetic fibers and plastics and the different types of synthetic fibers and plastics available.
3. To explore the various applications of synthetic fibers and plastics in different industries such as fashion, automotive, construction, and electronics.
4. To investigate the environmental impact of synthetic fibers and plastics and the importance of recycling and proper disposal methods.
5. To evaluate the advantages and disadvantages of synthetic fibers and plastics compared to natural materials and their impact on society and the economy.
6. To analyze the future trends and innovations in the field of synthetic fibers and plastics and their potential impact on different industries and the environment.
I need help please on chemistry

Answers
The image shows a representative sample of 50
atoms of a fictious element, called lemonium ( Le ). Lemonium consists of three isotopes. The red spheres are Le– 19 , the light blue spheres are Le– 17 , and the dark blue spheres are Le– 15. Determine the percent natural abundance of each of the three isotopes.
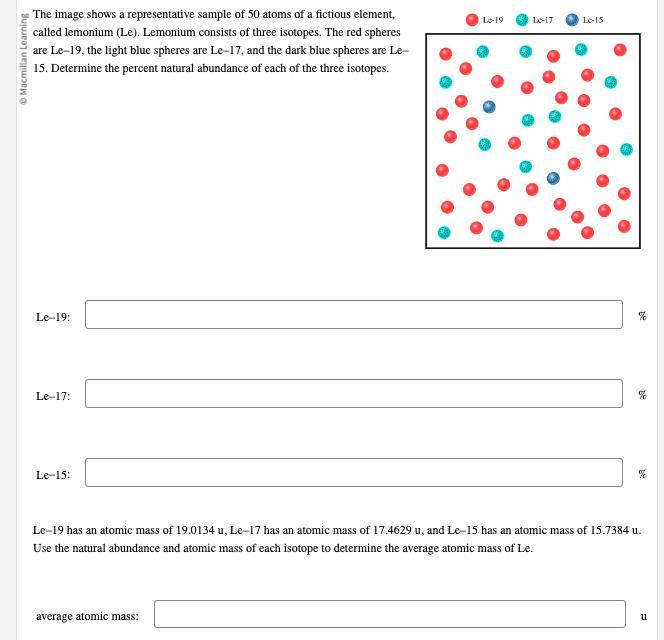
Answers
The percent natural abundance of Le-19, Le-17, and Le-15 isotopes are 40%, 44%, and 16% respectively.
Lemonium (Le) has three isotopes, which are Le-19, Le-17, and Le-15.
The given image shows a representative sample of 50 atoms of Lemonium, and we are to determine the percent natural abundance of each of the three isotopes.
Lemonium is an element having three isotopes, so we need to calculate the percent natural abundance of each of the three isotopes of Lemonium.
The percent natural abundance of the isotopes can be calculated as follows:Percent natural abundance of Le-19:As we know that Lemonium (Le) has three isotopes, so it can be represented as follows: Le-19, Le-17, and Le-15.
We are given that the number of Le-19 isotopes in the representative sample is 20.
So, the percentage of Le-19 isotopes can be calculated as follows:Percentage of Le-19 = (Number of Le-19 isotopes / Total number of Lemonium atoms) x 100% = (20/50) x 100% = 40%.
Therefore, the percent natural abundance of Le-19 is 40%.
Percent natural abundance of Le-17:Similarly, the number of Le-17 isotopes in the representative sample is 22.
So, the percentage of Le-17 isotopes can be calculated as follows:Percentage of Le-17 = (Number of Le-17 isotopes / Total number of Lemonium atoms) x 100% = (22/50) x 100% = 44%.
Therefore, the percent natural abundance of Le-17 is 44%.
Percent natural abundance of Le-15:Moreover, the number of Le-15 isotopes in the representative sample is 8.
So, the percentage of Le-15 isotopes can be calculated as follows:Percentage of Le-15 = (Number of Le-15 isotopes / Total number of Lemonium atoms) x 100% = (8/50) x 100% = 16%.
Therefore, the percent natural abundance of Le-15 is 16%.
For more such questions on isotopes
https://brainly.com/question/14220416
#SPJ8
a student thinks she might have accidentally mixed up her sugar and her salt and put them in the wrong containers the crystals look similar and have no smell which test can the student run to determine which one is sugar and which one is salt
A: Iodine test
B: Bendicts test
C: Indicator test
D: vinegar test
please help
Answers
a is the correct answer
Identify the arrows that represent the process of cooling.
liquid
gas
solid
Answers
The arrows B, C, and E represent the process of cooling.
Solid state: In the solid state, the molecules are arranged in a regular and fixed pattern. The molecules in a solid are closely packed that is the solid particles can not move.
Liquid state: In the liquid state, the molecules are present in an irregular pattern. The molecules are closely packed but particles can move.
Gaseous state: In the gaseous state, the molecules are present in an irregular manner. The molecules of gases are not closely packed and can move freely.
Therefore, the solid changes to liquid on heating, and the liquid changes to solid after cooling.
Liquid changes to a gaseous state on heating while gases changes to liquid on cooling.
Solid changes to gas on heating and gases change to solid after cooling.
To learn more about the process of cooling, visit: https://brainly.com/question/4385546
#SPJ9
Assume that 0.491 g of diborane is combusted in a calorimeter whose heat capacity (Ccalorimeter) is 7.854 kJ/°C at 19.63°C. What is the final temperature of the calorimeter?
Answers
Answer:
The combustion of diborane (B2H6) is as follows:
2B2H6(g) + 6O2(g) → 4H2O(g) + B4O(g)
The balanced chemical equation shows that 2 moles of B2H6 react with 6 moles of O2 to produce 4 moles of H2O and 1 mole of B4O. We can use this information to calculate the amount of heat released by the combustion of 0.491 g of B2H6:
0.491 g B2H6 × (1 mol B2H6/27.67 g B2H6) × (1 mole B4O/2 moles B2H6) × (-2037 kJ/mol B4O) = -7.89 kJ
The negative sign indicates that the reaction releases heat.
The heat released by the reaction is absorbed by the calorimeter, which causes its temperature to increase. We can use the equation:
q = Ccalorimeter × ΔT
where q is the amount of heat absorbed by the calorimeter, Ccalorimeter is the heat capacity of the calorimeter, and ΔT is the change in temperature of the calorimeter.
Rearranging the equation, we get:
ΔT = q/Ccalorimeter
Substituting the values we obtained, we get:
ΔT = (-7.89 kJ)/(7.854 kJ/°C) = -1.005°C
The negative sign indicates that the temperature of the calorimeter decreases by 1.005°C. Therefore, the final temperature of the calorimeter is:
19.63°C - 1.005°C = 18.625°C
Rounding to the appropriate number of significant figures, the final temperature of the calorimeter is 18.6°C.
Hope this is what you are looking for.
A rock displaces 1.65 L of water. The volume of the rock is:
Answers
Answer:
If a rock displaces 1.65L of water, its volume must be 1.65L.
If you want to convert that to cm^3 it's 1000 cm^3 per liter...so 1650 cm^3
Explanation:
Photosynthesis is a process that A green plants use to produce food. B animals use to breathe. C predators use to catch their prey. D illustrates symbiosis.
Answers
Answer: A
Explanation: Plants us photosynthesis transform sunlight, water, and carbon dioxide into oxygen, and simple sugars that the plant uses as fuel
To burn the mixture of methane CH4 and ethene C2H4, 10 mol of O2 are needed. How many moles of C2H4 are there in the mixture?
Answers
There are 3.33 moles of \(C_{2}H_{4}\) in the mixture.
The balanced chemical equation for the combustion of methane and ethene is:
\(CH_{4} + 2O_{2} = CO_{2} + 2H_{2}O\\C_{2}H_{4} + 3O_{2} = 2CO_{2} + 2H_{2}O\)
From the equation, we can see that for every mole of \(C_{2}H_{4}\), 3 moles of O2 are needed. Therefore, to burn 10 moles of O2, we need:
10 moles \(O_{2}\)x (1 mole \(C_{2}H_{4}\)/3 moles \(O_{2}\)) = 3.33 moles \(C_{2}H_{4}\)
So, there are 3.33 moles of \(C_{2}H_{4}\) in the mixture.
Learn more about moles, here:
https://brainly.com/question/29724957
#SPJ1