Which of the following will not be deflected by charged plates?
O Cathode rays
O Alpha particles
O Protons
O Hydrogen
Answers
Answer:
hydrogen
Explanation:
cathode rays, alpha particles and protons are charged particles so they will get deflected. Hydrogen consist of a single proton and single electron so it is neutral. therefore hydrogen will not get deflected
Related Questions
PLEASE HURRY!!!! The diagram shows 2 identical charged particle. How does the potential energy and the force of repulsion changes as the charges are brought closer together

Answers
Answer:
Please did you ever get an answer??
Explanation:
Im doing usa test prep too and its really late
How many grams of naoh are needed to prepare 546 ml of solution with a ph of 10. 00?.
Answers
Answer:
1. determining the pOH of the solution from the given pH of 10.00 assuming the solution is at 25° C.
We are studying the ideal gas law. In this discussion, you will be trying your hand at applying one of the ideal gas laws to a real world situation. Consider a situation that involves an ideal gas law and discuss how you would apply your chosen ideal gas law to the situation. Generate an ideal gas law question based on this situation.
Please do not forget to generate a question.
Answers
The ideal gas law, which relates the pressure, volume, temperature, and number of moles of an ideal gas, can be applied to real-world situations. By considering a specific scenario and applying the ideal gas law, we can analyze the behavior of gases and make predictions about their properties.
Let's consider a situation where a scuba diver is exploring underwater at a depth of 30 meters. We can apply the ideal gas law, specifically the form known as Boyle's law, which states that the pressure and volume of a gas are inversely proportional at constant temperature.
Question: How does the pressure of the gas in the scuba tank change as the diver descends to a depth of 30 meters, assuming the temperature remains constant?
To answer this question, we can use the ideal gas law equation PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature. By keeping the temperature constant, we can observe the relationship between pressure and volume as the diver descends and calculate the change in pressure based on the change in volume.
To learn more about Boyle's law click here:
brainly.com/question/30367133
#SPJ11
The Earth is in a protected area in the solar system where it does not get struck by asteroids.
True
False
Answers
Answer:
TRUEExplanation:
The ozone layer acts as a shield for life on Earth. Ozone is good at trapping a type of radiation called ultraviolet radiation, or UV light, which can penetrate organisms' protective layers, like skin, damaging DNA molecules in plants and animals.
for centuries scholars have argued over how to (select a word) certain cryptic passages in milton’s plays and poems.
Answers
for centuries scholars have argued over how to explicate certain cryptic passages in Milton’s plays and poems.
What is a cryptic passages?Cryptic Passage (originally titled as Passage to Transylvania) is described as the first expansion pack for Blood developed by Sunstorm Interactive and released in June 30, 1997.
The first of Blood's two expansion packs is called Cryptic Passage.
The expansion, unlike the Plasma Pak, was created by a third party, Sunstorm Interactive, and it was released by WizardWorks Software; as a result, it doesn't have any bug patches or new features.
Learn more about Cryptic Passage at:
https://brainly.com/question/27211381
#SPJ4
HELP PLEASE , I NEED A A ON THE TEST I’ll give brain

Answers
Answer:
left
Explanation:
8 has the strongest force
During each step of the electron transport system, electrons move to a more electronegative carrier, and thus move ______.
Answers
During each step of the electron transport system, electrons move to a more electronegative carrier, and thus move closer to a more stable state of energy.
Electrons are transferred to more electronegative carrier molecules during the electron transport chain (ETC) of cellular respiration. This creates an electron gradient across the membrane that can be used to produce ATP energy molecules. In the mitochondria of eukaryotic cells, the electron transport chain takes place. The electron transport chain includes several carriers of electrons that are membrane-bound.
NADH and FADH2 transfer electrons and hydrogen ions to the electron transport chain carriers during cellular respiration. These electrons then pass from one carrier molecule to the next, which allows the carriers to pump protons from the mitochondrial matrix to the intermembrane space. This sets up an electrochemical gradient that leads to the creation of ATP by the enzyme ATP synthase.
To know more about Electrons refer to:
https://brainly.com/question/26084288
#SPJ11
Use the reaction shown l to answer these questions.
2CO(g) + 2NO) → N2(g) + 2CO2(g)
If 42.7 g of CO is reacted completely at STP, what
volume of N2 gas will be produced?

Answers
Answer:
16.8dm3
Explanation:
2 moles of CO gives 1 mole of N2
2 moles of CO= 2* 28= 56g
1 mole of N2 = 1* 22.4dm
56g of CO gives 22.4dm3 of N2
42.7 of CO will give> (42.7*22.4)/56
=16.8dm3
The volume of \(N_{2}\) gas will be produced 16.8 \(dm^{3}\).
What is volumeVolume is sometimes referred to as capacity. The volume of a cylindrical jar, for example, is used to determine how much water it can hold.
Calculation of volume is shown below:
The given reaction is: 2CO(g) + 2NO) → N2(g) + 2CO2(g)
It can be seen that 2 moles of CO give 1 mole of \(N_{2}\).
So, 2 moles of CO = 2×28 = 56 g.
Hence, 1 mole of \(N_{2}\) = 1×22.4 = 22.4 \(dm^{3}\).
56 g of CO will give 22.4 \(dm^{3}\) of nitrogen atom.
42.7 of CO will give = (42.7×22.4) / 56
= 16.8 \(dm^{3}\).
Therefore, the volume of nitrogen will be 16.8 \(dm^{3}\).
To know more about volume click here.
https://brainly.com/question/1578538.
#SPJ2
atoms are so small that approximately _______ of them can fit at the end of a needle?
Answers
Answer:
5 million million atoms can fit at the end of a needle
Answer:
Let's just say 5 million million hydrogen atoms could fit at the end of a needle.
Explanation:
Atoms are 100 picometers, and to compare, the amount of even 1 centimeter, a calculator can't even figure out how many picometers are in a centimeter because there is just too much picometers.
identify the best explanation for which cation, lithium or sodium, has stronger ion-dipole interactions with water and why.
Answers
Lithium, as a stronger ion-dipole interaction results from a smaller atomic radius.
Atomic radius is that?The number of miles from a nuclear charge to its outermost electron orbital is typically defined as the atomic radius. Only by determining the separation seen between nuclei of two contacting molecules and halving a certain distance can one determine the dimension of an atom.
What factors affect atomic radius?The type of chemical bond that the atoms are engaged in determines the value of their atomic radii (metallic, ionic, or covalent bond). A portion of the observable distance separating atoms is attributed to one type of atom and the remainder to another type when the neighboring atoms are not identical, as in sodium chloride.
To know more about atomic radius visit:
https://brainly.com/question/17926486
#SPJ1
what does genetic modification produce
Answers
Answer:
Genetic modification or GMOs can change foods. It's typically used to preserve foods, enlargen foods or create seedless grapes
Explanation:
What atomic or hybrid orbitals make up the sigma bond between s and cl in sulfur dichloride, scl2?
Answers
Two Cl-atoms form a sigma bond with sp3 hybrid orbitals. Thus, SCl2 has sp3 hybridization.
The Lewis structure shows us that the carbon atom makes 4 sigma bonds to hydrogen and has no non-bonding electron pairs. The central carbon atom combines its 2s, 2px, 2py, and 2pz valence orbitals to make four, 2sp3 hybrid orbitals. Each one of these combines with a 1s atomic orbital from a hydrogen atom.
What is hybridization of SCl2?
In its most stable state, Sulfur acts as the central atom and forms two covalent bonds with the Chlorine atoms. It also possesses two lone pairs. Due to the presence of 4 electron domains and its steric number being 4, the hybridization of SCl2 is given by sp3.
To learn more about hybrid orbitals here
brainly.com/question/24195443
#SPJ4
What mass of the calcium carbonate, to the nearest hundredth of a gram, is decomposed in this reaction.

Answers
Answer:
28.00 g is the answer
Answer:200
Explanation:
It’s is the answer
Lab: Limiting Reactant and Percent Yield
Student Guide
Pre-Lab Information
Purpose Explore the yield of a chemical reaction by identifying the limiting reactant, comparing the
theoretical and actual yields, and explaining the sources of error.
Time Approximately 45 minutes
Question While observing a chemical reaction, how can you tell which reactant is limiting?
Reaction The reaction of copper(II) chloride and aluminum is shown in this balanced equation:
3CuCl2 + 2Al 2AlCl3 + 3Cu
Hypothesis If a substance is the limiting reactant, then it will be fully consumed by the time the
reaction completes because it is the reactant that reacts completely and the reaction
cannot proceed further.
Summary You will react copper(II) chloride with different quantities of aluminum in two trials. You
will also calculate percent yield for Trial 2.
Answers
Answer:
pre lab information would be the thing you to ressearch the lab to get the information
Limiting Reactant and Percent Yield Lab Report attached
Q1 Define and differentiate between the following: i. Temporary and permanent hardness
ii. Organic, ortho and poly phosphorus in wastewater
iii. Self-cleansing and scouring velocity in sewers iv. Type 1 and Type 2 settling in water/wastewater treatment v. Chloramines and Disinfection by-products
Answers
Temporary and permanent hardness of water Temporary hardness of water is caused by the presence of bicarbonate, carbonate, and sulfate ions, while permanent hardness is caused by the presence of chlorides, sulfates, and nitrates.
Carbonate and bicarbonate hardness can be removed using a process called boiling. Permanent hardness, on the other hand, can be removed using a process called ion exchange.ii. Organic, ortho, and polyphosphorus in wastewaterOrganic phosphorus is present in wastewater in the form of organic molecules like DNA, RNA, and phospholipids. Orthophosphate is the most common form of phosphorus found in wastewater. Polyphosphates, which are a chain of orthophosphate molecules, can also be found in wastewater.iii. Self-cleansing and scouring velocity in sewersSelf-cleansing velocity is the minimum velocity of wastewater flow required to prevent the deposition of solids in the sewer. Scouring velocity, on the other hand, is the minimum velocity required to remove previously deposited solids. Scouring velocity is higher than self-cleansing velocity.
Type 1 and Type 2 settling in water/wastewater treatment Type 1 settling occurs when particles of different sizes and densities settle separately, forming distinct layers. In type 2 settling, particles of different sizes and densities settle together in a mixed floc. Type 1 settling is more effective at removing larger particles, while type 2 settling is better at removing smaller particles.v. Chloramines and disinfection by-products (DBPs)Chloramines are a combination of chlorine and ammonia that are used as a disinfectant in water treatment. Disinfection by-products (DBPs) are formed when chlorine reacts with organic matter in the water. Some common DBPs include trihalomethanes (THMs) and haloacetic acids (HAAs), which are known to be carcinogenic.
To know more about Temporary hardness visit:-
https://brainly.com/question/31835462
#SPJ11
2) If you
move 50 meters in 10 seconds, what is your velocity?
Answers
Answer:
5 meter/second.
Explanation:
You dived the seconds, 10, by the meters, 50.
Chemistry
How many Fluorine ions are there in 202 grams of CaF2
HINT: Find the percentage of Fluorine in Calcium Fluoride, then find out how many grams of fluorine are in 200 grams of Calcium Fluoride. Lastly solve for ions
Answers
There are 48.72 g Fluorine ions
Further explanationProust stated the Comparative Law that compounds are formed from elements with the same Mass Comparison so that the compound has a fixed composition of elements
In the same compound, although from different sources and formed by different processes, it will still have the same composition/comparison
%F in CaF₂ :
\(\tt \%F=\dfrac{2.Ar~F}{MW~CaF_2}\times 100\%\\\\\%F=\dfrac{2.19}{78}\times 100\5=48.72\%\)
mass of Fluorine :
\(\tt 48.72\%\times 200=97.44~g\)
So mass Fluorine ions(2 ions F in CaF₂⇒Ca²⁺+2F⁻) :
\(\tt =\dfrac{97.44}{2}=48.72~g\)
Describe briefly how you would
determine the density of an irregular
Object
Answers
Answer:
See below
Explanation:
First determine the mass (weigh it) ..... then place it in a graduated container of water to see the volume of the object (by the amount of water it displaces)
then density = mass / volume
If a gas has a volume of 3.67 L and a pressure of 790 mm Hg, what will the pressure be if the volume is compressed to 2.12 L? What is the pressure in atmospheres (atm)?
Answers
Answer:
0.600
Explanation:
2.120/3.670 = 0.578 so 790*0.578 = 456.349 when converted to atm that is 456.349/760 = V2
0.600
If a gas has a volume of 3.67 L and a pressure of 790 mm Hg, the pressure if the volume is compressed to 2.12 L is 1367 mm Hg.
What is Boyle's law?The combined gas law is the law of of gaseous state which is made by combination of Boyle's law, Charle's law, Avogadro's law and Gay Lussac's law.
It is a mathematical expression that relates Pressure, Volume and Temperature.
(P1 × V1)÷T1 = (P2 × V2)÷T2
Boyle's law states that if temperature is constant then product of pressure and volume is constant.
(P1 × V1)= (P2 × V2)
Given,
P1 = 790 mm Hg
V1 = 3.67L
P2 = ?
V2 = 2.12 L
(P1 × V1)= (P2 × V2)
P2 = 1367 mm Hg
Therefore, If a gas has a volume of 3.67 L and a pressure of 790 mm Hg, the pressure if the volume is compressed to 2.12 L is 1367 mm Hg.
Learn more about Boyle's Law, here:
https://brainly.com/question/30367067
#SPJ2
5.26 kg of nitrogen monoxide and 7.64 mg of oxygen are combined, what mass of nitrogen dioxide is formed?2NO+O2=2NO2
Answers
Answer:
0.0220 g (22 mg) of NO2.
Explanation:
To solve this type of problem, it is best to work with grams. Remember that 1 kg equals 1000 g and 1 g equals 1000 mg. The conversion for 5.26 kg of nitrogen monoxide (NO) would be:
\(5.26\text{ kg NO}\cdot\frac{1000\text{ g}}{1\text{ kg }}=5260\text{ g NO.}\)And for 7.64 mg of oxygen (O2) is:
\(7.64\text{ mg O}_2\cdot\frac{1\text{ g}}{1000\text{ mg}}=0.00764\text{ g O}_2.\)The next step is to find the number of moles of each reactant using its molar mass. The molar mass of NO is 30 g/mol (you can calculate the molar mass of a compound using the periodic table):
\(5260\text{ g NO}\cdot\frac{1\text{ mol NO}}{30\text{ g NO}}=175.33\text{ moles NO.}\)And the moles of oxygen (O2) is 32 g/mol:
\(0.00764\text{ g O}_2\cdot\frac{1\text{ mol O}_2}{32\text{ g O}_2}=2.388\cdot10^{-4}mole\text{s O}_2.\)The next step is to see how many moles of NO" can be produced for each reactant.
You can see in the chemical equation that 2 moles of NO produce 2 moles of NO2, so the molar ratio between them is 1:1. This means that 175.33 moles of NO reacted produce 175.33 moles of NO2.
Now, you can see that 1 mol of O2 reacted produces 2 moles of NO2, so let's see how many moles of NO2 are being produced:
\(2.388\cdot10^{-4}mole\text{s O}_2\cdot\frac{2\text{ moles NO}_2}{1\text{ mol O}_2}=4.776\cdot10^{-4}mole\text{s NO}_2.\)You can note that the limiting reactant, in this case, is oxygen (O2) because this reactant imposes the "limit" to produce the product.
The final step is to convert from 4.776 x 10^(-4) moles of NO2 to grams using its molar mass which is 46 g/mol. The conversion will look like this:
\(4.776\cdot10^{-4}mo\text{les NO}_2\cdot\frac{46\text{ g NO}_2}{1\text{ mol NO}_2}=0.0220\text{ g NO}_2.\)We obtain 0.0220 g (22 mg) of NO2 from 5.26 kg of NO and 7.64 mg of O2.
Wenner four poles equal method is used to measure the soil resistivity near a 66/11 kV substation using a AEMC 6472 Ground Tester. The readings are recorded at 1, 2, 3, 4 and 5 m intervals of the probe distance. The corresponding soil resistance were measured to be 16.4, 5.29, 3.05, 1.96 and 1.36 2, respectively. Calculate the average soil resistivity in that substation.
Answers
The average soil resistivity near the substation is approximately 5.612 Ω·m.
To calculate the average soil resistivity near the substation, we can use the Wenner four poles equal method and the given soil resistance readings.
The formula for calculating soil resistivity using the Wenner method is:
ρ = (π * spacing * sum of resistance) / (2 * π * probe length)
Where:
ρ = Soil Resistivity
spacing = Distance between the current electrodes (m)
sum of resistance = Sum of the measured soil resistance values (Ω)
probe length = Length of the probe (m)
In this case, the probe distance intervals are 1, 2, 3, 4, and 5 m, and the corresponding soil resistance values are 16.4, 5.29, 3.05, 1.96, and 1.36 Ω, respectively.
Let's calculate the average soil resistivity:
spacing = 1 m (since the distance between the current electrodes is not mentioned, we assume it to be 1 m)
sum of resistance = 16.4 + 5.29 + 3.05 + 1.96 + 1.36 = 28.06 Ω
probe length = 5 m (as given in the intervals)
Using the formula, we have:
ρ = (π * spacing * sum of resistance) / (2 * π * probe length)
= (π * 1 * 28.06) / (2 * π * 5)
= 5.612 Ω·m
Therefore, the average soil resistivity near the substation is approximately 5.612 Ω·m.
Learn more about Wenner method from the given link!
https://brainly.com/question/31715647
#SPJ11
PLZ HELP!
What would happen to work and power if the mass of the person walking was increased or decreased? EXPLAIN.
Answers
Answer:
it would decrease / increase with the mass
Explanation:
Excessive use of chemical fertilizer cause chemical pollution.How?
Answers
Explanation:
Chemical fertilizers raise crop yields, but their heavy usage has hardened the soil, diminished fertility, reinforced pesticides, contaminated air and water, and emitted greenhouse gases, posing health and environmental risks.
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
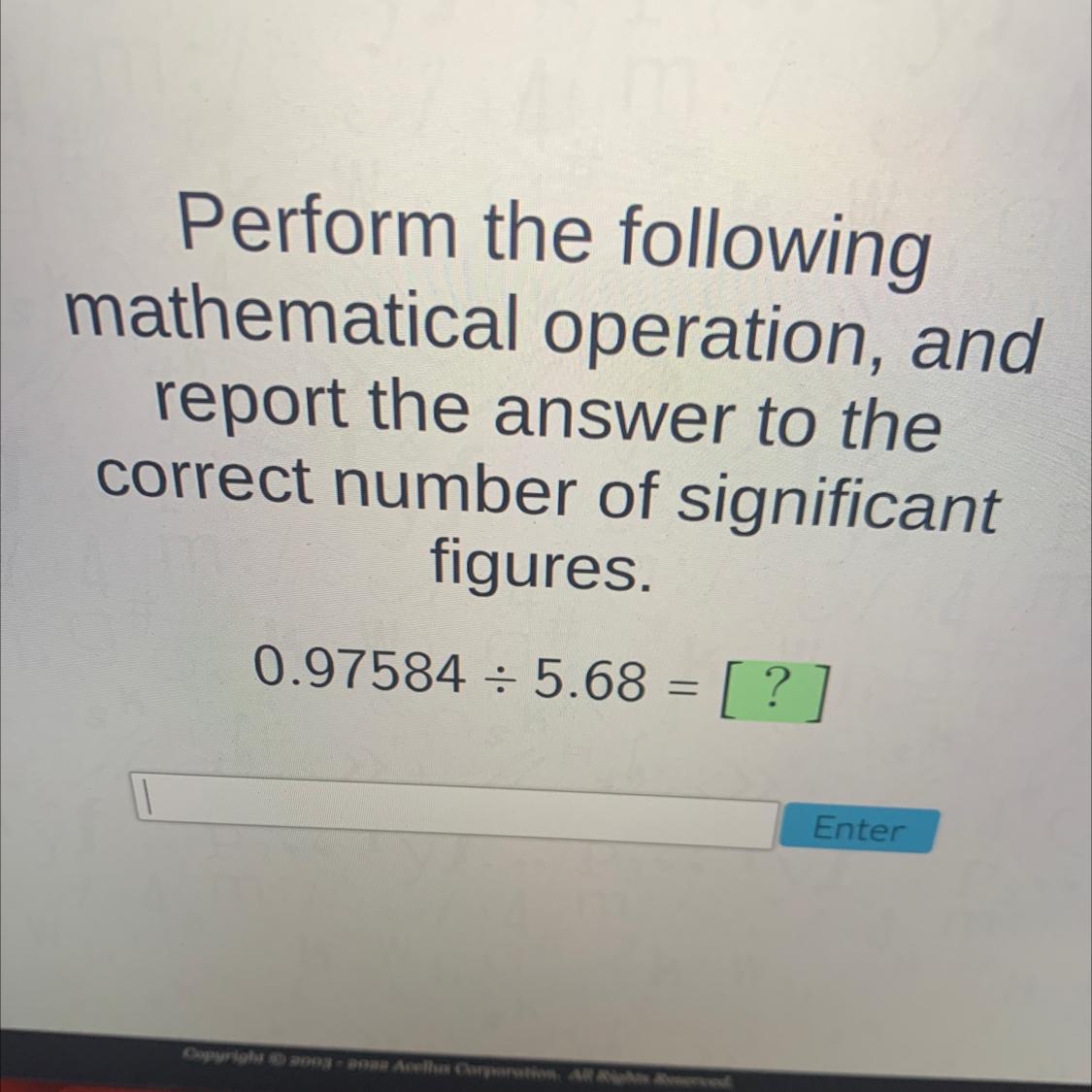
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Tectonic plate movement is responsible for forming which type of rock? Choose all that apply
Answers
Answer:
Regional metamorphism occurs at convergent plate boundaries, due to intense pressure. As two plates collide, the Earth's crust folds and faults. The intense pressure changes large areas of the Earth's crust into metamorphic rock. Mountain ranges are typically metamorphic rock, due to plate tectonic processes.
Explanation:
Hope this helps :D
Tectonic plate movement is responsible for forming regional metamorphic rock.
What is tectonic plate movement?The tectonic plate movement involves the subsurface movement of formation caused by natural force of earth. These movement can be uplifting, faulting or folding.
There are three main types of tectonic plate movement.
Divergent - The plate move apart from each other .Convergent - The plate move toward each other.Transform - The plate slide past each other.There are mainly three types of rock.
Igneous rock - They are formed from cooling of magma.Metamorphic rock - They are formed through change in igneous and sedimentary rock.Sedimentary rock - They are formed through solidification of sediments.Thus, tectonic plate movement is responsible for forming regional metamorphic rock.
To learn more about tectonic plate movement, refer to the below link:
https://brainly.com/question/16944828
#SPJ2
Your question is incomplete but most probably your full question was
Tectonic plate movement is responsible for forming which type of rock? Choose all that apply
A. Igneous rock
B. Metamorphic rock
C. Sedimentary rock
D. None of the above
another potential future fuel is methanol ( ch3oh ). enter a balanced chemical equation for the combustion of gaseous methanol. express your answer as a chemical equation
Answers
The balanced chemical equation for the combustion of gaseous methanol (CH₃OH) is: 2CH₃OH(g) + 3O₂(g) → 2CO₂(g) + 4H₂O(g)
The balanced chemical equation represents the combustion of gaseous methanol (CH₃OH) in the presence of oxygen (O₂). The coefficients in front of the chemical formulas indicate the relative number of molecules or moles involved in the reaction, ensuring that the equation is balanced in terms of mass and charge.
In the combustion reaction, two molecules of gaseous methanol combine with three molecules of oxygen gas. Through the combustion process, the methanol is oxidized, and carbon dioxide (CO₂) and water (H₂O) are produced as the main products.
The balanced equation shows that two molecules of methanol react with three molecules of oxygen to yield two molecules of carbon dioxide and four molecules of water.
This balanced equation represents the stoichiometry of the combustion reaction, ensuring that the number of atoms of each element is the same on both sides of the equation.
To know more about combustion, refer here:
https://brainly.com/question/14335621#
#SPJ11
What is the solubility of silver iodide in grams per milliliter at a temperature at which the Kₛₚ of Agl is 1.47 x 10 ⁻¹⁶?
Answers
The solubility product constant expression for AgI is:
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
The Ksp expression for AgI is given as 1.47 x 10⁻¹⁶.
Since AgI dissociates into 1 Ag⁺ ion and 1 I⁻ ion, the molar solubility (s) of AgI is equal to the concentration of Ag⁺ and I⁻ ions in the solution.
Let's assume the molar solubility of AgI is s M.
Since the molar solubility (s) of AgI is equal to the concentration of Ag⁺ and I⁻ ions, we have:
[Ag⁺] = s M
[I⁻] = s M
Using the stoichiometry of the balanced equation, the expression for the solubility product constant is:
Ksp = [Ag⁺][I⁻] = s^2
Substituting the given Ksp value, we have:
1.47 x 10⁻¹⁶ = (s)^2
Taking the square root of both sides, we get:
s = √(1.47 x 10⁻¹⁶)
Calculating the square root, we find:
s ≈ 3.83 x 10⁻⁹ M
Since the solubility is given in grams per milliliter (g/mL), we need to convert the molar solubility to grams per milliliter using the molar mass of AgI.
The molar mass of AgI is:
Ag: 107.87 g/mol
I: 126.90 g/mol
AgI: 107.87 g/mol + 126.90 g/mol = 234.77 g/mol
To convert the molar solubility (s) to grams per milliliter (g/mL):
s (g/mL) = (molar solubility (M) * molar mass of AgI (g/mol)) / 1000
Substituting the values, we have:
s (g/mL) = (3.83 x 10⁻⁹ M * 234.77 g/mol) / 1000
Calculating the value, we find:
s (g/mL) ≈ 9.0 x 10⁻¹² g/mL
Therefore, the solubility of silver iodide (AgI) in grams per milliliter (g/mL) at the given temperature is approximately 9.0 x 10⁻¹² g/mL.
The solubility of silver iodide (AgI) in grams per milliliter can be calculated using the concept of solubility product constant (Kₛₚ). Given that the Kₛₚ of AgI is 1.47 x 10⁻¹⁶.
The solubility product constant (Kₛₚ) is a measure of the equilibrium between a solid and its dissolved ions in a saturated solution. For silver iodide (AgI), the equilibrium equation can be expressed as:
AgI(s) ⇌ Ag⁺(aq) + I⁻(aq)
The Kₛₚ expression for this equilibrium is:
Kₛₚ = [Ag⁺][I⁻]
Given the Kₛₚ value of AgI as 1.47 x 10⁻¹⁶, it indicates that the product of the concentrations of Ag⁺ and I⁻ ions in the saturated solution is equal to 1.47 x 10⁻¹⁶.
To determine the solubility of AgI in grams per milliliter, we need to know the molar mass of AgI and the volume of the saturated solution. The molar mass of AgI is 234.77 g/mol, which is the sum of the atomic masses of silver (Ag) and iodine (I).
To convert the concentration of Ag⁺ or I⁻ ions to grams per milliliter, we need to divide the concentration (in moles per liter) by the molar mass (in grams per mole) and multiply by the solution volume (in milliliters).
However, without the given volume of the saturated solution, it is not possible to calculate the solubility in grams per milliliter directly using the Kₛₚ value. The solubility information typically depends on both temperature and the presence of other ions or substances in the solution. Therefore, additional data or an experimental approach would be needed to determine the solubility of AgI in grams per milliliter at the given temperature.
To learn more about solubility - brainly.com/question/32565143
#spj11
In a simple distillation setup, what is the sequence of equipment from the bench top to the round bottom flask
Answers
In a simple distillation setup, the sequence of equipment from the bench top to the round bottom flask is:
ThermometerDistillation flaskLiebig condenserRound bottom flaskBunsen burnerWhat is Distillation?This is the process in which a mixture is separated through selective boiling and condensation.
The distillation flask and liebig condenser are usually located above the round bottom flask in the set up.
Read more about Distillation here https://brainly.com/question/24553469
#SPJ4
Some calcifiers can use bicarbonate (HCO3-) to make their shells.
How will they be affected by the decrease in pH?

Answers
Answer:
The removal of this ion from water by the calcifiers will definitely decrease the pH of water.
What is the bicarbonate ion?
The bicarbonate ion is the ion that is designated as HCO3-. We must notice that this ion is present in abundant amounts in the sea. This amount definitely make the sea slightly alkaline.
Now we must note that the removal of this ion from water by the calcifiers that require it to make their shell will definitely decrease the pH of water.
Explanation:
The removal of this ion from water by the calcifiers will definitely decrease the pH of water.
What is the bicarbonate ion?The bicarbonate ion is the ion that is designated as \(HCO^{3-}.\) We must notice that this ion is present in abundant amounts in the sea. This amount definitely makes the sea slightly alkaline.
Now we must note that the removal of this ion from water by the calcifiers that require it to make their shell will definitely decrease the pH of water.
Hence, the removal of this ion from water by the calcifiers will definitely decrease the pH of water.
Learn more about the bicarbonate ion here:
https://brainly.com/question/13164182
#SPJ5
What is the strongest intermolecular force present in HCl?
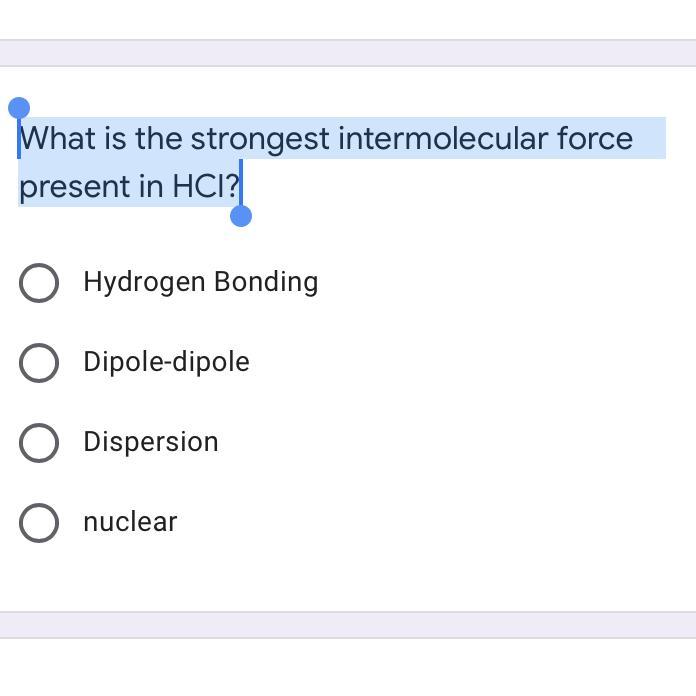
Answers
Answer:
Dipole dipole interaction
Explanation: