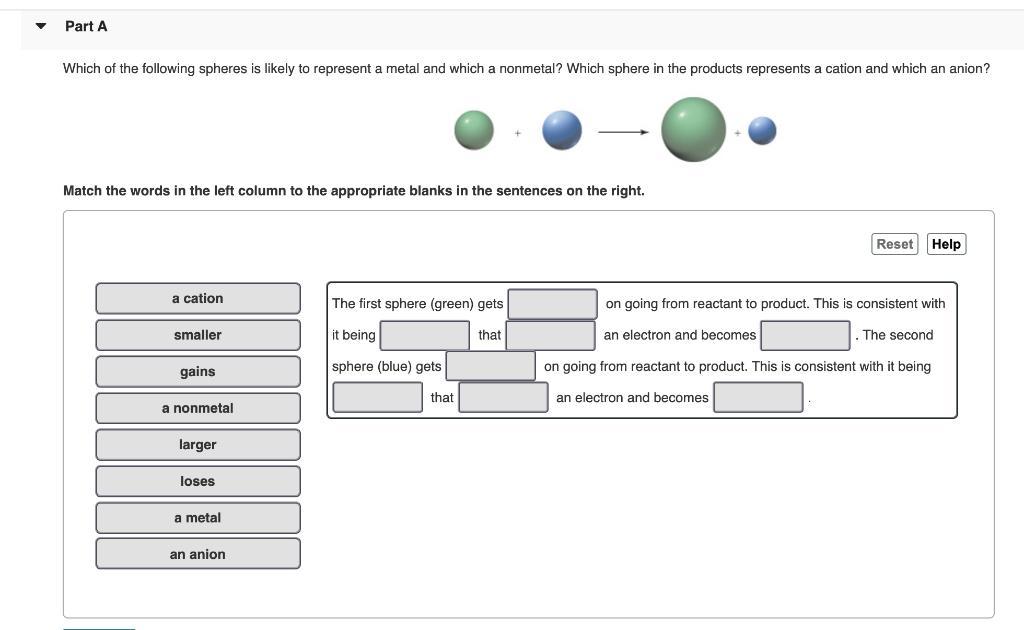
which of the following spheres is likely to represent a metal and which a nonmetal? which sphere in the products represents a cation and which an anion?
Answers
Since cations are smaller than their parent atoms (metals form cations), the first (red) sphere represents the metal atom. Since anions are bigger than their parent atoms (nonmetals), the second (blue) sphere represents the nonmetal atom.
A metal is any of a group of substances with high thermal and electrical conductivity, malleability and ductility, and high light reflectivity. Metals make up roughly 75% of all chemical elements that are currently understood. In the Earth's crust, aluminum, iron, calcium, sodium, potassium, and magnesium are the most prevalent elements. Some metals, such as copper, gold, platinum, and silver, are frequently found in the free state, though, as a result of the fact that they do not easily react with other elements. Ores contain the majority of metals (materials that contain minerals). Typically, metals are crystalline solids. They typically have a highly symmetric crystal structure with a compact atom packing that is simple to understand.
To know more about metal visit : https://brainly.com/question/1349130
#SPJ4
Related Questions
according to the third law of thermodynamics,40)a)the entropy of the universe increases for any spontaneous process.b)energy is conserved in any transformation of matter.c)the entropy of a perfectly ordered, crystalline substance is zero at 0 kelvin.d)the entropy increases for any spontaneous process.
Answers
In keeping with the third regulation of thermodynamics the entropy of a perfectly ordered, crystalline substance is 0 at 0 Kelvin.(choice c).
The legal guidelines of thermodynamics are a hard and fast of medical legal guidelines that outline groups of physical portions which include temperature, electricity, and entropy that represent a thermodynamic machine in thermodynamic equilibrium.
The 0th law of thermodynamics defines thermal equilibrium and bureaucracy the basis for the definition of temperature. If structures are in thermal equilibrium with a third gadget, they're in thermal equilibrium with every different.
The primary regulation of thermodynamics states that after energy enters or leaves a device (as paintings, heat, or depend), the internal power of the gadget changes in keeping with the law of conservation of electricity.
The second one law of thermodynamics states that during a herbal thermodynamic system the total entropy of interacting thermodynamic systems by no means decreases. the overall implication of this declaration is that warmness does no longer spontaneously transfer from a cold frame to a warm body.
The 0.33 regulation of thermodynamics states that the entropy of a gadget procedures a constant value as the temperature tactics absolute zero. The entropy of a machine at absolute zero is usually near zero, except for amorphous solids.
Learn more about law of thermodynamics here:
https://brainly.com/question/26035962
#SPJ4
fallacies we don't need to make election day a holiday. we have had the elections for over two centuries and we have never made election day a holiday.
Answers
The type of fallacy that this excerpt exemplifies is an appeal to tradition (third option) rather than a false dichotomy, an appeal to pity, or the strawman fallacy.
What fallacy does the speaker use in this excerpt?Remember that a fallacy takes place if the reason provided for an argument is not valid as it is not supported by facts. This occurs in the statement presented because the reason for the speaker to support the idea of not having the elections a holiday is that this has never been done before.
This is not a valid reason and it is only based on tradition, therefore it can be categorized as an appeal to tradition.
Note: This question is incomplete; here are the missing options:
Group of answer choices
False dichotomy
Appeal to Tradition
Strawman Fallacy
Appeal to Pity
Learn more about fallacies in https://brainly.com/question/30761126
#SPJ4
Which of the following would allow you to find the volume of an irregular shaped solid object?
a: triple beam balance
b: l × b × h
c: water displacement
d: hight × length × width
Answers
Answer:
water displacement
that is c
Which describes a feature of synthetic polymers? They can be naturally decomposed. They can be cheaply recycled. They can only be made in small quantities. They can be produced at a low cost.
Answers
Answer:
The correct option is;
They can be produced at low cost
Explanation:
In comparison to several other manufacturing materials, some synthetic polymers such as plastics are more durable cost effective, flexible, faster to produce and reliable
With regards to the cost comparison, the resins used for plastic are cheaper to obtain than mining for materials such as metals. In all quantities, it is cheaper to produce and manufacture plastics than to produce metals.
Due to the light weight and corrosion resistance of plastics, it is cheaper to store and transport plastics to several locations with different environment without the plastic requiring much effort for transportation or being affected by corrosion.
Answer:
d
Explanation:
Help me please…………..d

Answers
Diffusion in Solids It is desired to calculate the rate of diffusion of CO₂ gas in air through a loosely packed bed of sand at 276K and a total pressure of 1 atm. The bed depth is 1.25 m and the void fraction e is 0.3. The partial pressure of CO₂ at the top of the bed is 2.026 x 10' Pa and 0 Pa at the bottom. Assume equimolar counterdiffusion of CO₂ and air. Use a t of 1.87. DAB-0.142×10 m²/s.
Answers
the rate of diffusion of CO₂ gas in air through the bed of sand is approximately 2.304 × 10^-6 mol/(m²·s).
To calculate the rate of diffusion of CO₂ gas in air through a bed of sand, we can use Fick's law of diffusion:
J = -DAB (dC/dx)
where J is the molar flux of CO₂, DAB is the diffusion coefficient of CO₂ in air, and (dC/dx) is the concentration gradient of CO₂ in the direction of diffusion.
To calculate the concentration gradient, we can use the following equation:
(dC/dx) = (ΔC/Δx)
where ΔC is the difference in partial pressure of CO₂ between the top and bottom of the bed, and Δx is the bed depth.
We are given that the bed depth is 1.25 m and the void fraction is 0.3, which means that the volume of the bed is:
V = (1 - e) A L
where A is the cross-sectional area of the bed and L is the bed depth. Assuming a circular cross-section, we can calculate the area as:
A = π (d/2)²
where d is the diameter of the bed. We are not given the diameter, so we cannot calculate the area.
However, we are given the partial pressure of CO₂ at the top and bottom of the bed, as well as the diffusion coefficient and temperature. We can use these values to calculate the molar flux of CO₂ using Fick's law of diffusion.
First, we need to convert the diffusion coefficient to the appropriate units:
DAB = 0.142 × 10^-9 m²/s
Next, we can calculate the concentration gradient:
ΔC = 2.026 × 10^4 Pa - 0 Pa = 2.026 × 10^4 Pa
Δx = 1.25 m
(dC/dx) = (ΔC/Δx) = (2.026 × 10^4 Pa/1.25 m) = 1.6208 × 10^4 Pa/m
Finally, we can calculate the molar flux of CO₂:
J = -DAB (dC/dx) = -(0.142 × 10^-9 m²/s) (1.6208 × 10^4 Pa/m) = -2.304 × 10^-6 mol/(m²·s)
The negative sign indicates that the molar flux of CO₂ is in the opposite direction of the concentration gradient, which is expected for equimolar counterdiffusion.
Therefore, the rate of diffusion of CO₂ gas in air through the bed of sand is approximately 2.304 × 10^-6 mol/(m²·s).
if yu had 1.73 moles of hydrogen (h2) and 0.89 moles of oxygen (o2), which is the limiting reactant?
Answers
Since 0.865 is smaller than 0.89, hydrogen (H2) is the limiting reactant in this reaction.
To determine the limiting reactant, we need to compare the moles of each reactant to the stoichiometric ratio of the balanced equation. The balanced equation for the reaction between hydrogen and oxygen to form water is:
2H2 + O2 -> 2H2O
According to this equation, 2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water.
To determine which reactant is limiting, we can use the mole ratio of the reactants in the equation. For every 1 mole of oxygen, we need 2 moles of hydrogen. So, for 0.89 moles of oxygen, we would need 1.78 moles of hydrogen.
Since we only have 1.73 moles of hydrogen, it is the limiting reactant. This means that all 0.89 moles of oxygen will react completely with 1.73 moles of hydrogen, and any remaining hydrogen will be left over after the reaction is complete.
More on limiting reactant: https://brainly.com/question/16974583
#SPJ11
All matter has both physical and chemical properties. A physical property can be observed without changing the identity of the substance. Which is a physical property?
A. ability to rust
B. density
C. flammability
D. reactivity with the water
Answers
Explanation:
Density
Hope it helps :)
Which is not found on the periodic table of elements. the atomic number of each element, the rarity of each element, the atomic mass of each element
Answers
Answer:
The rarity of each element.
Explanation:
The periodic table shows the atomic number and the atomic mass. The abundance (or rarity) of each element is not given.
Answer: sorry misclick
Explanation:
How many moles of copper are in 3.22g of copper?
Answers
Answer:
I hope it helps even though I don't think it's much

According to the mole concept, there are 0.050 moles of copper in 3.22 g of copper.
What is a mole?Mole is defined as the unit of amount of substance . It is the quantity measure of amount of substance of how many elementary particles are present in a given substance.
It is defined as exactly 6.022×10²³ elementary entities. The elementary entity can be a molecule, atom ion depending on the type of substance. Amount of elementary entities in a mole is called as Avogadro's number.
It is widely used in chemistry as a suitable way for expressing amounts of reactants and products.For the practical purposes, mass of one mole of compound in grams is approximately equal to mass of one molecule of compound measured in Daltons. Molar mass has units of gram per mole . In case of molecules, where molar mass in grams present in one mole of atoms is its atomic mass.
Number of moles is calculated as, mass/molar mass
Hence on substitution in above formula, number of moles= 3.22/63.54=0.050 moles,
Thus, there are 0.050 moles in 3.22 g of copper.
Learn more about moles,here:
https://brainly.com/question/20486415
#SPJ2
why do we say the partials in a rock lying on the ground have kinetic energy and potential energy
Answers
Answer:
All particles of matter are always in constant motion. In this case, the particles of the rock possess kinetic energy as they vibrate in place. However, the particles also contain potential energy due to their position and arrangement. This form of stored energy is responsible for keeping the particles bonded together.
Explanation:
A small plastic cup is floating in a pan of water. Inside the cup is one quarter. For an experiment, students take turns adding quarters to the cup one quarter at a time. Making observations after each quarter is added to the cup, the students will notice something about the water level in the pan. What do they notice about the water level?
A: The cup will sink
B: The cup will go up
C: The level will go down
D: The level will remain the same
i dont know why is says high school im in middle school
Answers
Additionally, the water level will rise.
Answer:
Explanation:
A
Which metalloids would behave more like metals? Which metalloids would behave more like nonmetals?.
Answers
The metalloid that tends to act more like metals is boron.
The metalloids that tend to act more like nonmetals are arsenic, antimony, and tellurium.
Metals are the elements that have the ability to lose electrons, while nonmetals are the elements with the ability to gain electrons.
Metalloids, Metals, and NonmetalsThe metalloids are the elements with the ability to act as both metals and nonmetal. The metalloids have wide application, with its characteristic in between metals and nonmetals.
The metalloids with less than 4 electrons in the valence shell tends to act like metals, while metalloids with more than 4 valence electrons tends to act more like nonmetals.
The metalloid that tends to act more like metals is boron.
The metalloids that tend to act more like nonmetals are arsenic, antimony, and tellurium.
Learn more about metalloids, here:
https://brainly.com/question/17482288
Answer: A. metalloids in group 16 and B. metalloids in group 13
Explanation: took the test and got it right
How do body cells in multicellular organisms reproduce?
O A. Through mitotis
B. Through gamete fusion
O C. Through sexual reproduction
O D. Through meiosis
Answers
Answer:
meiosis, mitosis
Explanation:
well if your asking for the cells, they split into 2 via mitosis. This is known as cell division which is what creates new body cells. But if you are talking about meiosis it makes the components for sexual reproduction in multicellular organisms like humans, who then sexually reproduce. Meiosis creates egg and sperm cells.
The body cells in multicellular organisms reproduce through mitosis and through meiosis. The correct options are A and D.
What are multicellular organisms?Cells in multicellular organisms are specialized to carry out particular tasks. For instance, red blood cells in humans are designed specifically to provide oxygen and nutrients to all bodily cells.
Additionally, germinal gametic cells divide by meiosis, whereas somatic cells of multicellular animals divide via mitosis.
Cell division, which is what produces new body cells, is this process. Meiosis, on the other hand, creates the elements needed for sexual reproduction in multicellular creatures like humans, who subsequently engage in sexual reproduction.
Therefore, the correct options are A. Through mitosis, and D. Through meiosis.
To learn more about multicellular organisms, refer to the below link:
https://brainly.com/question/24381583
#SPJ2
Germanium has 5 isotopes. The average atomic mass from the periodic table is 72.63.
A)Find the missing abundance.
B) Use the atomic mass to calculate the mass of the missing isotope.

Answers
Answer:
75.92
Explanation:
A)the % abundance adds up to 100, so adding all the % abundances of Ge:
20.52 + 27.45 + 7.76 + 36.52 = 92.25
The remaining % abundance is:
100 - 92.25 = 7.75%
B) Using this formula:
\(atomic \: mass = \frac{sum \: of \: (\% \: isotope \: abundance \times isotope \: mass)}{100} \)
\(72.63 = \frac{(20.52 \times 69.92) + (27.45 \times 71.93) + (7.76 \times 72.92) + (36.52 \times 73.92) + (7.75x)}{100} \)
multiply both sides by 100 to get rid of the fraction:
\(7263= {(20.52 \times 69.92) + (27.45 \times 71.93) + (7.76 \times 72.92) + (36.52 \times 73.92) + (7.75x)}{} \)
multiplying each bracket:
\(7263 = 1434.7584 + 1974.4785 + 565.8592 + 2699.5584 + 7.75x\)
adding up the terms:
\(7263 = 6674.6545 + 7.75x\)
subtract 6674.6545 on both sides to give:
\(588.3455 = 7.75x\)
divide both sides by 7.75:
\(x = \frac{588.3455}{7.75} = 75.92 \: (2dp)\)
what could you do to increase the electrical force between two charged particles by a factor of 16
Answers
Increasing one charged particle by 16 will lead to increasing the electrical force between two charged particles by a factor of 16.
What is Electrical force?This is referred the attractive or repulsive interaction between any two charged bodies present.
F= kq1q2/r²
F1/F2= kq1q2/r² / 16kq1q2/r²
F1/F2 = 1/16
F2 = 16F1
This is therefore how to increase the electrical force between two charged particles by a factor of 16.
Read more about Electrical force here https://brainly.com/question/1498868
#SPJ1
Based on the pKa of benzoic acid and the pKa of HCl, what do you hypothesize will happen in terms of reaction equilibrium? a) the equilibrium will favor the starting materials (sodium benzoate and HCl) because HCl has a lower pKa b) the equilibrium will favor the starting materials (sodium benzoate and HCl) because benzoic acid has a lower pKa c) the equilibrium will favor the products (benzoic acid and NaCl) because benzoic acid has a lower pKa d) the equilibrium will favor the products ( benzoic acid and NaCl) because HCl has a lower pKa
Answers
The equilibrium will favor the products (benzoic acid and NaCl) because HCl has a lower pKa. The correct answer is (d).
The pKa of HCl is -7, while the pKa of benzoic acid is 4.2. This means that HCl is a much stronger acid than benzoic acid.
When benzoic acid reacts with sodium hydroxide (NaOH), it forms sodium benzoate and water. This reaction is a type of acid-base reaction, where benzoic acid acts as an acid and NaOH acts as a base.
When sodium benzoate is treated with hydrochloric acid (HCl), it reacts to form benzoic acid and sodium chloride (NaCl). This reaction is also a type of acid-base reaction, where sodium benzoate acts as a base and HCl acts as an acid.
The equilibrium of the reaction between sodium benzoate and HCl will depend on the relative strengths of the two acids. Since HCl is a much stronger acid than benzoic acid, it will be more likely to react with sodium benzoate than benzoic acid will be to react with NaOH.
Therefore, the equilibrium will favor the products (benzoic acid and NaCl) because HCl has a lower pKa. The correct answer is (d).
Learn more about reaction equilibrium here: https://brainly.com/question/15118952
#SPJ11
(e) A 0.050 mol sample of a hydrocarbon was burned in excess oxygen.
The products were 3.60 g of water and 6.60 g of carbon dioxide.
(i) Calculate the number of moles of carbon dioxide produced.
Relative atomic masses: C = 12; O = 16.
Moles of carbon dioxide =
*
(2)
Answers
The correct answer is 0.15.
We are aware that there is 0.05 mol of an unidentified hydrocarbon we will refer to as "X" and that its burning produces 6.6 g of carbon dioxide and 3.6 g of water.
These quantities might be converted to moles by applying the following formula:
amount= mass/ relative atomic mass
Thus, the following equation may be written for H2O: moles = 3.6 / 18 = 0.2 and for CO2: moles = 6.6 / 44 = 0.15.
0.05X + x'O2 = 0.15CO2 + 0.2H2O
This may be made simpler by dividing through by 0.05 (this step is likely to be the most helpful to you), resulting in:
1 x + x O2 = 3 co2 + 4 H2O
The hydrocarbon must have been the source of all the carbon in the carbon dioxide and all the hydrogen in the water.
Accordingly, 4 x 2 = 8 moles of H and 3 x 1 = 3 moles of C.
There are 3/1 = 3 Cs and 8/1 = 8 Hs in one X molecule.
This clearly identifies C3H8 or propane as the hydrocarbon X (dividing by 1 seems unnecessary, but it illustrates the process to use if there were more than one mol of X in the first equation).
To learn more about number of moles of carbon dioxide refer the link:
https://brainly.com/question/12723070
#SPJ9
Please select the word from the list that best fits the definition
a new energy source found in the ocean that is currently very costly to obtain
nonrenewable resources
renewable resources
methane hydrates
plankton
petroleum
nonliving resources
Answers
Answer:renewable resources
Explanation:
Atomic masses of element
Hydrogen and carbon
Answers
Answer:
Atomic mass of Hydrogen is 1.00784 u
Atomic mass of Carbon is 12.0107 u
Explanation:
which of the following correctly describes the basic chemical formula for all sugar monomers?
Answers
The basic chemical formula for all sugar monomers can be described as (CH₂O)n, where "n" represents the number of carbon atoms in the sugar molecule.
Glucose is the most prevalent natural monomer among sugars. Starch, cellulose, and glycogen combine to create polymers as a result of their linking. Many species rely on glucose as a critical source of energy.
This formula indicates that sugars are composed of carbon (C), hydrogen (H), and oxygen (O) atoms in a ratio of 1 ratio 2 ratio 1. The "n" value varies depending on the specific sugar monomer. For example monosaccharide, glucose, a common sugar monomer, has a chemical formula of C₆H₁₂O₆, where "n" is 6.
Therefore, the correct description of the basic chemical formula for all sugar monomers is (CH₂O)n.
To know more about monosaccharide:
https://brainly.com/question/30548064
#SPJ4
A Type la supernova results from the explosion of a former high mass stare fusion of hydrogen on the outside of a white dwarf. FEEDBACK: If a white dwarf accretes enough mass to enlarge it beyond 1.4 solar masses, the degenerate carbon core will not be able to resist gravitational collapse and the star will fuse carbon in a thermonuclear ruriaway explosion-a Type La supernova (page 322. A Stellar Cataclysm). See also Figure 12.19 (page 321). collision between a White dwaef and its bistafy companion
Answers
The supernova explosion of Type 1a supernovae is so bright that they are used as "standard candles" in astronomy.
A Type la supernova results from the explosion of a former high mass star, fusion of hydrogen on the outside of a white dwarf and collision between a White dwarf and its binary companion. Feedback suggests that if a white dwarf accretes enough mass to enlarge it beyond 1.4 solar masses, the degenerate carbon core will not be able to resist gravitational collapse and the star will fuse carbon in a thermonuclear runaway explosion, a Type La supernova.
Type 1a supernova results from the explosion of a white dwarf star that has gained additional mass and become unstable. A white dwarf is a dense core that remains after the outer layers of a star have been ejected during a nova. They are believed to exist in binary star systems, where they capture gas from their companion.
If the white dwarf gains enough mass, the carbon core is forced into rapid fusion, releasing a tremendous amount of energy and causing the star to explode. The supernova explosion of Type 1a supernovae is so bright that they are used as "standard candles" in astronomy.
To learn more about supernova ,
https://brainly.com/question/28375022
#SPJ4
How many gram of olid alumnuim ulfied can be prepared by the reaction of 10. 0 gram of alumnium and 15. 0 gram of ulfur?how much of the non limiting
reactant in exce?
Answers
The mass of \(Al_{2} S_{3}\) produced is 15.616 grams.
The reaction taking place is as follows:
\(2Al+ 3S\) →\(Al_{2} S_{3} (s)\)
Moles of Al = mass/molar mass = 10.0g/27.0g/mol
= 0.370 mol
Moles of S = mass/molar mass = 15.0g/(32.065g/mol)
= 0.468 mol
Al and S reacts in the molar ratio of 2:3.
2 moles of Al reacts with 3 moles of S
0.370 moles of Al will react with S = (3/2)*0.370mol
= 0.555 mol
Similarly, 0.468 moles of S will react with Al = 2/3 *0.468mol
= 0.312 mol
Thus, Al is in excess and S is the limiting reactant (some of Al will be left over ,S will completely react)
So, moles of \(Al_{2} S_{3}\) produced=1/3*0.312 mol of S
= 0.104 mol
Mass of \(Al_{2} S_{3}\) produced = moles*molar mass of \(Al_{2} S_{3}\)
= 0.104mol*150.158g/mol
= 15.616 grams
To learn more about limiting reactants, here
https://brainly.com/question/14225536
#SPJ4
How many atoms are in 2 moles of Ca
Answers
Mole is a unit of measurement used to measure the amount of a chemical substance, with 1 mole containing 6.022 x 1023 atoms, or 1.204 x 1024 atoms.
What is a mole?A mole is a unit of measurement in chemistry that is used to determine the quantity of a chemical. It is described as the quantity of a material containing the same number of particles (atoms, molecules, ions, and so on) as there are atoms in 12 grammes of carbon-12.
2 moles of Ca have 2 x 6.022 x 1023 atoms. This is due to the fact that the mole (mol) is a unit of measurement used to determine the quantity of a chemical compound. 1 mol of a material corresponds to 6.022 x 1023 atoms of that substance. As a result, 2 moles of Ca contain 2 x 6.022 x 1023 atoms, or 1.204 x 1024 atoms.
To know more about mole, visit
brainly.com/question/26416088
#SPJ1
One atom has 11 protons and 13 neutrons. Another has 11 protons and 12 neutrons. Are they the same or different elements? Explain your answer
Answers
Answer: Both the given species are isotopes of the same element.
Explanation:
An isotope is defined as the chemical species that have the same atomic number but a different mass number.
The atom consists of 3 subatomic particles:
Protons: They are positively charged particles present in the nucleus of an atom. Electrons: They are negatively charged particles present in the orbits around the nucleus of an atom. Neutrons: They are neutral particles present in the nucleus of an atom.An atomic number is defined as the number of protons or number of electrons present in a neutral atom. It remains specific for a particular element.
Mass number is defined as the sum of the number of protons and neutrons present in the nucleus of an atom.
Given values:
For isotope 1:
Number of protons = 11 = atomic number
Number of neutrons = 13
Mass number = 11 + 13 = 24
For isotope 2:
Number of protons = 11 = atomic number
Number of neutrons = 12
Mass number = 11 + 12 = 23
As the atomic number of both the isotopes is the same. Thus, they belong to the same elements.
What we call the factor a scientist
changes to observe its effects?
Answers
What is the uncertainty (in % RSD) of a 1.00ppm standard solution prepared by pipetting 10uL of a 1000 ppm (s
Answers
The % RSD of a 1.00 ppm standard solution prepared by pipetting 10 µL of a 1000 ppm (stock solution) will be 0.1%. The relative standard deviation (RSD) is used to compare the variability in a set of measurements to the mean of the set.
It is the ratio of the standard deviation to the mean. RSD = (standard deviation / mean) × 100. The percentage RSD for a 1.00 ppm standard solution prepared by pipetting 10 µL of a 1000 ppm (stock solution) is calculated as follows: Concentration of the stock solution = 1000 ppm
Volume of the stock solution pipetted
= 10 µL Volume of the diluted solution made
= 1000 µL (10 µL × 100)
Concentration of the diluted solution = (1000 ppm × 10 µL) / 1000 µL
= 10 ppm
Relative standard deviation (%RSD)
= (Standard deviation / Mean) × 100
= (0.01 / 10) × 100 = 0.1%.
Hence, the % RSD of a 1.00 ppm standard solution prepared by pipetting 10 µL of a 1000 ppm (stock solution) is 0.1%.
To know more about relative standard deviation visit :
https://brainly.com/question/28561334
#SPJ11
2) 2KClO3 --> 2KCl + 3O2
a) How many moles of O2 are produced from 19 moles of KClO3?
b) How many kilograms of KClO3 would decompose to form 62 moles of KCl?
c) How many grams of O2 are required to react with 39 grams of KCl?
show work
Answers
\(2 \text{ KClO}_3 \to 2 \text{ KCl}+3\text{ O}_2\)
a)
\(2 \text{ mols of KClO}_3 \equiv 3 \text{ mols of O}_2\)
\(19 \text{ mols of KClO}_3 \equiv 3\cdot 9,5 \text{ mols of O}_2\)
\(\boxed{19 \text{ mols of KClO}_3 \equiv 28,5 \text{ mols of O}_2}\)
b)
\(2 \text{ mols of KClO}_3 \equiv 2 \text{ mols of KCl}\)
\(62 \text{ mol of KClO}_3 \equiv 62 \text{ mol of KCl}\)
Using the atomic mass given in the periodic table:
\(62\cdot(39+35,5+16\cdot3) \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(62\cdot122,5 \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(7595 \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(\boxed{7,595 \text{ kg of KClO}_3 \equiv 62 \text{ mol of KCl}}\)
c)
\(2 \text{ KCl}+3\text{ O}_2\to 2 \text{ KClO}_3\)
\(3 \text{ mols of O}_2 \equiv 2 \text{ mols of KCl}\)
Using the atomic mass given in the periodic table:
\(3\cdot(2\cdot 16) \text{ g of O}_2 \equiv 2\cdot(39+35,5) \text{ g of KCl}\)
\(96\text{ g of O}_2 \equiv 149\text{ g of KCl}\)
\(\dfrac{39}{149}\cdot 96\text{ g of O}_2 \equiv \dfrac{39}{149}\cdot 149\text{ g of KCl}\)
\(\boxed{25,13\text{ g of O}_2 \equiv 39\text{ g of KCl}}\)
This result is an aproximation.
An a-t base pair consists of _____ h-bond(s); a c-g base pair consists of _____ h-bond(s).
Answers
An a-t base pair consists of 2 h-bond(s); a c-g base pair consists of 3 h-bond(s). The correct option is E.
What are base pairs?
Base pairs are the pairs that are made up of nucleotides. They are pairs of DNA bases. The base pairs are connected with each other by hydrogen bonds.
In between adenine and thymine, there are two or double hydrogen bonds present and between cytosine and guanine, there are triple bonds present.
So, there is 2 hydrogen bonds and triple hydrogen bonds in the base pairs of DNA, the genetic material.
Thus, the correct option is E, 2, 3.
To learn more about base pairs, refer to the link:
https://brainly.com/question/17254975
#SPJ4
The question is incomplete. Your most probably complete question is given below:
A. 1, 2.
B. 2, 1.
C. 2, 2.
D. 3, 2.
E. 2, 3.
23.5 ml of a stock solution that has a concentration of 7.29 m is added to water until the total volume of the new solution is 458 ml. what is the concentration (in molarity) of the new solution? report your answer to the hundreths place and do not include units in your answer.
Answers
The volumetric flask should contain the prepared solution after it has been made.
What is the straightforward meaning of concentration?My focus is being disrupted by all the commotion. 1a: the act or process of concentrating: the state of being concentrated especially: direction of attention to a single thing The student decided to focus on law as his academic major or area of interest.
23.5ml of the 458 mol L-1 solution
The moles per liter unit is used to express the solution concentrations. Consequently, change the volume of the volumetric flask's unit to liters as follows:
23.5ml
=23.5ml * 1L/10 ML
Convert the unit of the required stock solution to milliliters:
6.40* 10-3 L * 10 ml/1L = 6.40 ml
To learn more about 'solution' refer to
https://brainly.com/question/24917200
#SPJ4