Which of the following reactions is irreversible?
A. Zinc (Zn) reacting with hydrochloric acid (HCl)
B. Haber Process
C. Dehydrating copper sulfate (CuSO4)
D. Thermal decomposition of NH4Cl
PLEASE HELP
Answers
Answer:
A. Zinc (Zn) reacting with hydrochloric acid (HCl)
Explanation:
The reaction of Zinc with hydrochloric acid is an irreversible chemical process.
What makes a reaction reversible;
it must be involved in reversible chemical processes, no matter how small the extent of the reversibility. the rate of forward reaction must be equal to the backward one.So, hydrochloric acid when reacting with Zinc will give a complete chemical change that will go into completion.
Answer:
(A): Zinc (Zn) reacting with hydrochloric acid (HCI)
Explanation:
I got it right on founders edtell
Related Questions
Changing the pressure of a gas is a way of changing the.
Answers
Changing the pressure of a gas is a way of changing the volume of the gas.
The volume of a gas is directly proportional to its pressure, meaning that as pressure increases, the volume of the gas decreases, and vice versa. This relationship is known as Boyle's law. Therefore, if you want to change the volume of a gas, you can do so by changing its pressure.
Changing the pressure of a gas can affect not only its volume but also its temperature and density. When you increase the pressure of a gas, you force its molecules closer together, which decreases the space between them and reduces the volume of the gas. Conversely, when you decrease the pressure of a gas, you allow its molecules to move further apart, which increases the space between them and increases the volume of the gas.
However, changing the pressure of a gas can also affect its temperature. When you compress a gas, you add energy to its molecules, which increases their kinetic energy and raises the temperature of the gas. Conversely, when you expand a gas, you remove energy from its molecules, which decreases their kinetic energy and lowers the temperature of the gas.
To know more about pressure, visit:
https://brainly.com/question/22613963
#SPJ11
carbon tetrachloride has been widely used in the cleaning industry in fire extinguishers and refrigerated. construct an explanation of how carbon and chlorine combine to form carbon tetrachloride
Answers
Answer:
The dense vapours of carbon tetrachloride forms a protective layer on the burning objects and avoids the oxygen or air to come in contact with the fire from the burning objects and provides incombustible vapours.
Explanation:
Hope This Helps
Happy Hoildays
~Zero~
How much 0.3 m h2so4 is needed to neutralize 34.0 ml of a 0.25 m solution of naoh?
Answers
14.1 mL H₂SO₄ is needed to neutralize solution of NaOH.
Balanced chemical equation for neutralization reaction of sulfuric acid and sodium hydroxide:
H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O
c(H₂SO₄) = 0.3 M = 0.3 mol/L; concentration of sulfuric acid
V(NaOH) = 34.0 mL = 0.034 L; volume of sodium hydroxide
c(NaOH) = 0.25 M = 0.25 mol/L; concentration of sodium hydroxide
n(NaOH) = c(NaOH) × V(NaOH)
n(NaOH) = 0.25 mol/L × 0.034 L.
n(NaOH) = 0.0085 mol; amount of sodium hydroxide
From chemical reaction: n(H₂SO₄) : n(NaOH) = 1 : 2.
n(H₂SO₄) = 0.0085 mol ÷ 2
n(H₂SO₄) = 0.00425 mol; amount of sulfuric acid
V(H₂SO₄) = n(H₂SO₄) ÷ c(H₂SO₄).
V(H₂SO₄) = 0.00425 mol ÷ 0.3 mol/L.
V(H₂SO₄) = 0.0141 L = 14.1 mL; volume of sulfuric acid
More about neutralization: brainly.com/question/23008798
#SPJ4
Which process describes the wearing
away of rock?
A. drainage
B. erosion
C. infiltration
D. weathering
Answers
Answer:
weathering
Explanation:
hope this helps
someone help please i cant figure this out
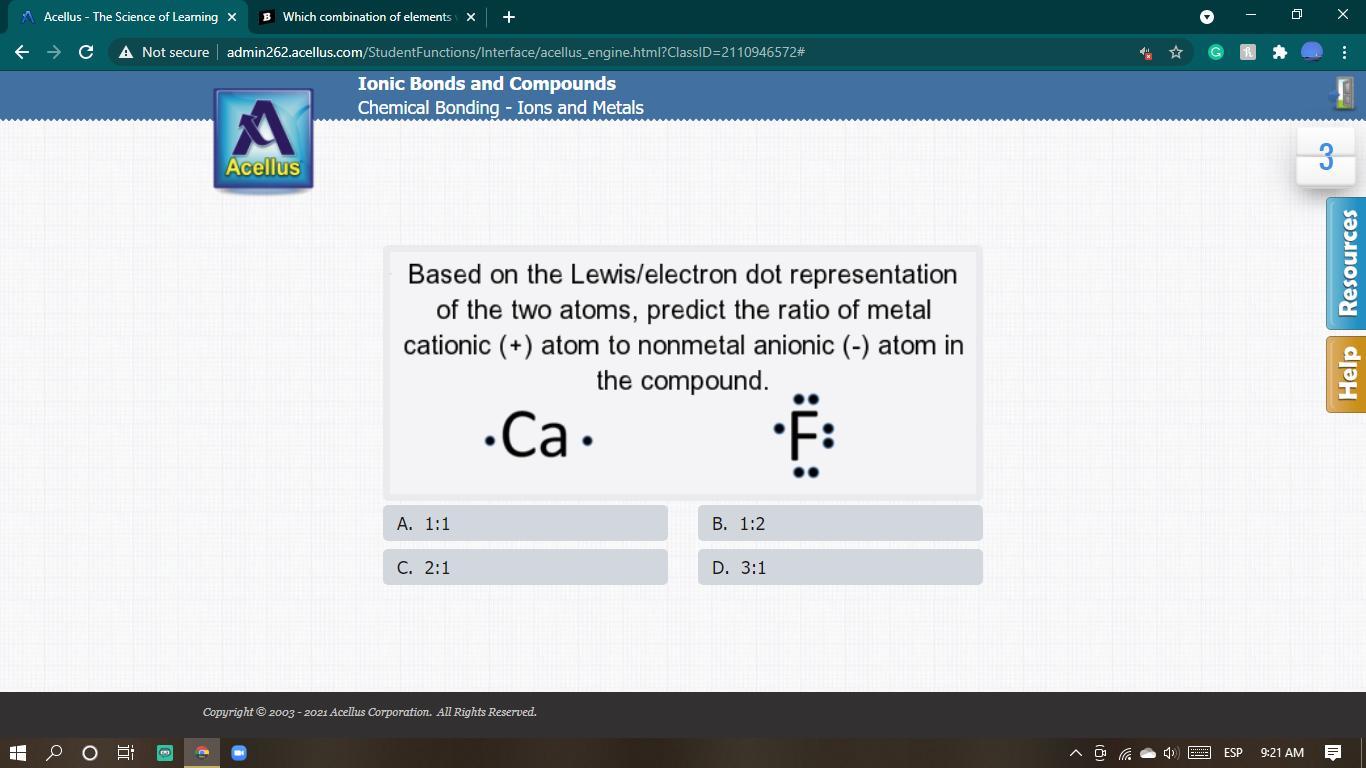
Answers
Answer: 2 : 1
Explanation:
Cation :
Ca - calcium = atomic number = 20
Electron dot configuration : 2, 8, 8, 2
Ca losses 2 electrons in its outermost shell and thus has a charge of 2+ in other to attain a stable octet state.
Anion:
F - has 7 valence electrons and thus needs 1 electron to achieve a stable octet state, hence it accepts one electron has has a charge of (-1)
Therefore,
Ratio of cation (+) to negative (-) = 2 :1
A particular mountain stream is home to a population of salamanders that are red in
color. In the past year, a second population of salamanders that are dark blue in color
moved into the same stream. Scientists researching the stream have recently discovered young salamanders that are red with dark blue patches on their bodies.
What can the scientists conclude from this evidence?
A). The salamanders are suffering from a bacterial disease.
B)..The salamanders are on the verge of becoming extinct.
C). The salamanders undergo asexual reproduction.
D). The salamanders undergo sexual reproduction.
Answers
Answer:
D
Explanation:
The salamanders undergo se×ual reproduction.
What is Se×ual reproduction?
This is defined as the production of new organisms by the combination of genetic information of two individuals of different genders.
Thus results in variations in the offsprings produced which us why the Salamanders had different color patches.
Read more about Se×ual reproduction here https://brainly.com/question/815744
9.Magnetization of iron is a physical change.
A true
B false
Answers
Answer:
This is true.
Explanation:
Magnetization of iron is a physical change and not a chemical change as there is no change of state, no change of temperature, no smell and no evolution of gas.
The total number of atoms in the formula aluminum acetate is
a. 15
b. 22
c. 9
d. 10
e. 13
will give branliest and 100 points I guess? please be quick though
Answers
Answer:
B) 22 atoms
Explanation:
Aluminum Acetate has a formula of C6H9AlO6, meaning that it has 6 Carbon atoms, 9 Hydrogen atoms, 1 Aluminum atom, and 6 Oxygen atoms.
In total there are: 6+9+1+6 atoms, or a total of 22 atoms.
Hope this helps!
Answer:
The molecular or chemical formula of Aluminium Acetate is C6H9AlO6.
6-carbon atoms
9-hydrogen atoms
6-oxygen atoms
1-aluminium atom
Therefore the total number of atoms in the formula aluminum acetate is (6+9+6+1)= 22 atoms.b. 22 is the right answer
how much energy is required to take ice from -15 C to 125 C
(150g of ice)
Answers
It takes approximately 406,687.5 joules of energy to take 150 grams of ice from -15°C to 125°C.
To determine the amount of energy required to take ice from -15°C to 125°C, we need to consider two stages of the process; Heating the ice from -15°C to 0°C, causing it to melt, and Heating the resulting water from 0°C to 125°C
We can calculate the amount of energy required for each stage separately and then add them together to get the total energy required.
Heating the ice from -15°C to 0°C; The specific heat capacity of ice is 2.09 J/(g·°C), which means that it takes 2.09 joules of energy to raise the temperature of 1 gram of ice by 1°C. Since we have 150 grams of ice, we can calculate the amount of energy required to raise the temperature of the ice from -15°C to 0°C as;
Q1 = m × c × ΔT
= 150 g × 2.09 J/(g·°C) × (0°C - (-15°C))
= 4,987.5 J
Therefore, it takes 4,987.5 joules of energy to heat the ice from -15°C to 0°C
Heating the water from 0°C to 125°C; The specific heat capacity of water is 4.18 J/(g·°C), which means that it takes 4.18 joules of energy to raise the temperature of 1 gram of water by 1°C. We need to heat the water from 0°C to 100°C (the boiling point of water at standard pressure) and then from 100°C to 125°C.
For the first stage, we can calculate the amount of energy required as;
Q₂a = m × c × ΔT
= 150 g × 4.18 J/(g·°C) × (100°C - 0°C)
= 62,700 J
The heat of vaporization of water at standard pressure is 2,260 J/g. Since we have 150 grams of water, we can calculate the amount of energy required to convert all the water to steam as:
Q₂b = m × Lv = 150 g × 2,260 J/g
= 339,000 J
Therefore, it takes a total of;
Q = Q₁ + Q₂a + Q₂b
= 4,987.5 J + 62,700 J + 339,000 J
= 406,687.5 J
Therefore, it takes 406,687.5 joules of energy.
To know more about heat of vaporization here
https://brainly.com/question/11465845
#SPJ1
A solution is 5.00% potassium chloride by mass. How much potassium chloride would you expect to collect by evaporating 150.0 g of the solution?

Answers
Answer:
C. 7.50g
Explanation:
The percent (%) by mass of a solute in a solution refers to the number of grams contained in 100g of solution by that solute. In this case, 5% by mass of pottasium chloride (KCl) means 5g of KCl is contained in 100g of solution.
Therefore, in 150g of solution, there would be:
5g/100g × 150g
= 0.05 × 150
= 7.50g of KCl solute.
Hence, 7.50g of pottasium chloride would be expected to be collected by evaporating 150.0 g of the solution.
why is it important to run a blank solution to set the zero %T for both Parts 1 and 11 in this experiment? How would your results be affected if you did not run a blank? 2. A student neglected to run the blank solution to set the zero %T in Part l and obtained the Beer's Law plot shown below. a. If the student used the plot as shown, how would their calculated values of Ke be affected b. How could the student modify their plot to improve their results? 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 10 20 30 40 concentration (M × 10°)
Answers
Running a blank solution is crucial in spectrophotometry experiments to establish the zero %T and account for background absorbance. Without running a blank, the results can be affected by systematic errors.
It is important to run a blank solution to set the zero %T in both Parts 1 and 2 of the experiment because it helps to account for any background absorbance or interference from the solvent or other components in the sample. Running a blank solution allows us to establish a baseline measurement of the solvent or the solution without the analyte, which helps in accurately measuring the absorbance caused by the analyte of interest.
If a blank solution is not run, the results can be affected in several ways:
Systematic Error: The absence of a blank solution can introduce a systematic error, causing a constant offset in the measured absorbance values. This offset can lead to incorrect calculations and interpretations.
Overestimation or Underestimation: Without running a blank, the measured absorbance may include contributions from the solvent or other interfering substances. This can lead to overestimation or underestimation of the analyte concentration, affecting the accuracy of the results.
Distorted Beer's Law Plot: In the absence of a blank, the plot obtained may not accurately represent the linear relationship between concentration and absorbance according to Beer's Law. This can lead to incorrect calculations of the slope (molar absorptivity) and affect the accuracy of future concentration determinations.
In spectrophotometry, the blank solution serves as a reference for setting the zero %T (transmittance) or absorbance value. By measuring the blank, we can account for any absorbance caused by the solvent, impurities, or other components in the sample. The blank solution typically contains all the components except the analyte of interest. It is measured under the same conditions as the sample solutions.
The blank measurement allows us to subtract any background absorbance from the sample measurements, providing a more accurate representation of the absorbance caused solely by the analyte. This helps in obtaining reliable and precise measurements for concentration determination using Beer's Law.
Running a blank solution is crucial in spectrophotometry experiments to establish the zero %T and account for background absorbance. Without running a blank, the results can be affected by systematic errors, inaccurate concentration determinations, and distorted Beer's Law plots. It is important to always include a blank solution to ensure accurate and reliable measurements.
To know more about spectrophotometry , visit:
https://brainly.com/question/24864511
#SPJ11
consider the chemical reaction: h2 (g) i2 (g) 2hi (g) at 721 k when you increase the order of the reaction what does happen to the half-life of the reaction?
Answers
When the order of the reaction increases the half-life of the reaction decreases. This is because the rate constant k becomes smaller as the order of the reaction increases, and the half-life is inversely proportional to k.
The given chemical reaction:
H₂(g) + I₂(g) → 2HI(g)
Is a second-order reaction, as the overall order of the reaction is 2 (sum of the orders of the reactants). The rate law for this reaction will be:
Rate = k[H₂][I₂]
where k is the rate constant, [H₂] and [I₂] are the concentrations of hydrogen and iodine gases, respectively.
The half-life of a second-order reaction is given by the equation:
\(t_{1/2}\) = 1 / k[A]0
where [A]₀ is the initial concentration of one of the reactants. In this case, we can take either [H₂]₀ or [I₂]₀ as the initial concentration.
Now, increasing the order of the reaction means changing the rate law by increasing the exponent of the concentration term. For example, if the reaction becomes third-order, the rate law would be:
Rate = k[H₂]²[I₂]
If the reaction becomes higher order, the rate law would be even more complex.
To know more about second-order reaction here
https://brainly.com/question/12446045
#SPJ4
What do the molecules do when they gain more kinetic energy and how does that affect the density?
Answers
The more kinetic energy a substance has, the warmer it will be and the faster particles will be moving, which reduces the density of the substance
what is the atomic number of an element based on
Answers
Answer:
The atomic number is the number of protons in the nucleus of an atom . The number of protons define the identity of an element
Calculate the molar mass AND identify the gas if 3.25 kg of this gas is stored in a 15.00 L tank and exerts a pressure of 120.0 atm at a constant temperature of 66.0°C.
Answers
Answer:
is your question correct
.
..
.
.
.
or not
which one of the following molecules is polar? group of answer choices ccl4 xef2 xef4 pbr5 brf5
Answers
Among the given molecules, the polar molecule is XeF2. XeF2, or xenon difluoride, has a polar covalent bond and an asymmetrical molecular geometry, making it a polar molecule.
The polarity of a molecule is determined by the difference in electronegativity between its constituent atoms and the overall molecular geometry. In XeF2, xenon (Xe) has a lower electronegativity compared to fluorine (F). As a result, the fluorine atoms exert a greater pull on the shared electrons, creating a partial negative charge (δ-) on each fluorine atom and a partial positive charge (δ+) on the xenon atom.
Additionally, the molecular geometry of XeF2 is linear, with the two fluorine atoms on opposite sides of the central xenon atom. This arrangement leads to an asymmetrical distribution of charge within the molecule, resulting in a net dipole moment and overall polarity.
In contrast, CCl4 (carbon tetrachloride), XeF4 (xenon tetrafluoride), PBr5 (phosphorus pentabromide), and BrF5 (bromine pentafluoride) have symmetrical molecular geometries and/or symmetrical charge distributions, resulting in a net dipole moment of zero. These molecules are considered nonpolar.
Know more about Polar Molecules here:
https://brainly.com/question/31023968
#SPJ11
Write the condensed structural formulas for all products of the reaction between 2-bromo-2-methylpropane and ch3-ch2-oh. name the reaction mechanism.
Answers
The reaction between 2-bromo-2-methylpropane (commonly known as tert-butyl bromide) and CH3-CH2-OH (ethanol) results in the formation of two products: tert-butyl ethyl ether and hydrogen bromide. The reaction mechanism involved is nucleophilic substitution.
1. Nucleophilic Substitution: Ethanol (CH3-CH2-OH) acts as a nucleophile and replaces the bromine atom in tert-butyl bromide (2-bromo-2-methylpropane) through a nucleophilic substitution reaction.
2. Product 1: The condensed structural formula for tert-butyl ethyl ether (also known as ethoxy-tert-butane) is (CH3)3COCH2CH3. This compound is formed when the ethoxy group (CH3-CH2-O-) of ethanol replaces the bromine atom in tert-butyl bromide.
3. Product 2: Hydrogen bromide (HBr) is formed as a byproduct of the reaction. Its condensed structural formula is HBr.
Therefore, the products of the reaction between 2-bromo-2-methylpropane and CH3-CH2-OH are tert-butyl ethyl ether [(CH3)3COCH2CH3] and hydrogen bromide (HBr). The reaction mechanism involved is nucleophilic substitution.
Learn more about nucleophilic substitution from the given link: https://brainly.com/question/30633020
#SPJ11
which of the following statements is not true?
A. covalent compounds have low melting and boiling points.
B. covalent bonds occur between nonmetals.
C. covalent compounds are often gases or liquids.
D. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules.
Answers
Answer:
I think option (D)is not true
49 grams of sulfuric acid, H2SO4, is dissolved in 1 liter of solution. Determine the molarity (M).
Answers
Answer: .5m
Explanation:
A house is 57.0ft ieng and 38.0ft wide and has B.0-ft-high celings. What is the volume of the interioe of the house in cubic meters and cubic centimeters? m3
Answers
Volume of the interior of the house in cubic meters = 487.1 m³
Volume of the interior of the house in cubic centimeters = 4.871 × 10^8 cm³
Given the dimensions of the house as 57.0ft length, 38.0ft width and 8.0ft high ceilings.
The volume of the house can be found by using the formula for the volume of a rectangular solid as:
V = lwh
where
V is the volume,
l is the length,
w is the width,
h is the height of the house
Given,
l = 57.0ft
w = 38.0ft
h = 8.0ft
Now, substituting these values in the formula for the volume of the house, we get;
V = lwh
= 57.0 ft × 38.0 ft × 8.0 ft
= 17248.0 cubic feet
We know that 1 cubic meter = 35.3147 cubic feet
Volume of house in cubic meters
V = 17248.0/35.3147 m³ = 487.1 m³
Thus, the volume of the interior of the house in cubic meters is 487.1 m³.
The volume of the interior of the house in cubic centimeters can be found by using the fact that 1 m³ = 10^6 cubic centimeters
Volume of the house in cubic centimeters = 487.1 × 10³ × 10^6= 4.871 × 10^8 cm³
Thus, the volume of the interior of the house in cubic centimeters is 4.871 × 10^8 cm³.
Volume of the interior of the house in cubic meters = 487.1 m³
Volume of the interior of the house in cubic centimeters = 4.871 × 10^8 cm³
Learn more about cubic centimeters from this link:
https://brainly.com/question/12776623
#SPJ11
for the given reaction, what volume of o2 would be required to react with 4.8 l of co , measured at the same temperature and pressure?
Answers
The volume of O₂ required to react with 4.8 L of CO, measured at same temperature and pressure is 2.4 L
How do I dtermine the volume of O₂ required?To obtain the volume of O₂ required, we shall consider the equation for the reaction, in order to obtain relevant information. This is shown below:
2CO(g) + O₂(g) -> 2CO₂(g)
From the balanced equation above,
2 L of CO required 1 L of O₂
With the above information, we can obtain the volume of O₂ required to react with 4.8 L of CO as follow:
From the balanced equation above,
2 L of CO required 1 L of O₂
Therefore
4.8 L of CO will require = (4.8 × 1) / 2 = 2.4 L of O₂
THus, the volume of O₂ required is 2.4 L
Learn more about volume:
https://brainly.com/question/9614052
#SPJ1
Complete question:
2CO(g) + O₂(g) -> 2CO₂(g)
for the given reaction, what volume of o2 would be required to react with 4.8 l of co , measured at the same temperature and pressure?
melt pool geometry and microstructure of ti6al4v with b additions processed by selective laser melting additive manufacturing
Answers
These factors contribute to the formation of a fine-grained microstructure, which can affect the material's mechanical properties.
The melt pool geometry and microstructure of Ti6Al4V with B additions processed by selective laser melting additive manufacturing can be explained as follows:
1. Melt pool geometry: When the Ti6Al4V alloy with B additions is processed using selective laser melting (SLM) additive manufacturing, a laser beam is used to selectively melt the metal powder layer by layer, resulting in the formation of a melt pool. The melt pool geometry refers to the shape and dimensions of this molten region.
2. Microstructure: The microstructure of a material refers to its internal arrangement of grains, phases, and other microstructural features. In the case of Ti6Al4V with B additions processed by SLM, the microstructure is influenced by the rapid solidification that occurs after the melting process. The cooling rate during SLM can lead to the formation of a fine-grained microstructure, which can have an impact on the material's mechanical properties.
3. Manufacturing: Selective laser melting (SLM) is an additive manufacturing process that involves building objects layer by layer using a laser to selectively melt metal powders. In the case of Ti6Al4V with B additions, SLM offers the advantage of producing complex shapes and structures with good mechanical properties.
4. 150: The number "150" mentioned in the question might refer to a specific parameter or condition related to the melt pool geometry and microstructure of Ti6Al4V with B additions processed by SLM. However, without further context, it is not possible to provide a specific explanation for this number.
In summary, the melt pool geometry and microstructure of Ti6Al4V with B additions processed by selective laser melting additive manufacturing can be influenced by factors such as the shape and dimensions of the melt pool, the rapid solidification process, and the cooling rate during SLM. These factors contribute to the formation of a fine-grained microstructure, which can affect the material's mechanical properties.
learn more about mechanical properties on :
https://brainly.com/question/29673011
#SPJ11
An object with a mass of 8.2 g raises the level of water in a graduated cylinder from 25.1 mL to 28.3 mL. What is the density of the object? g/mL
Answers
Answer:
The density is 2.5625g/mL. Hope this helped! :)
Explanation:
M= 8.2g D=M/V
V= 28.3-25.1=3.2mL
D=8.2g/3.2mL
D= 2.5625g/mL
Calculate the mass of a sample of gold that
occupies 0.242 cm3
. The density of gold is
19.3 g/cm3
.
Answer in units of g.
Answers
Answer:
4.6706g
Explanation:
Answer:
\(\boxed {\boxed {\sf 4.67 \ g }}\)
Explanation:
We are asked to calculate the mass of a sample of gold. We are given the density and volume.
Density is a substance's mass per unit volume. Therefore, the formula for calculating density is:
\(\rho= \frac{m}{v}\)
The density is 19.3 grams per cubic centimeter and the volume is 0.242 cubic centimeters. Substitute the values into the formula.
ρ=19.3 g/cm³v= 0.242 cm³\(19.3 \ g/cm^3 = \frac{m}{0.242 \ cm^3}\)
We are solving for the mass of the gold sample, so the variable m must be isolated. It is being divided by 0.242 cubic centimeters. The inverse operation of division is multiplication. Multiply both sides of the equation by 0.242 cm³.
\(0.242 \ cm^3 * 19.3 \ g/cm^3 = \frac{m}{0.242 \ cm^3} * 0.242 \ cm^3\)
\(0.242 \ cm^3 * 19.3 \ g/cm^3 = m\)
The units of cubic centimeters cancel.
\(0.242 * 19.3 \ g = m\)
\(4.6706 \ g = m\)
The original measurements of mass and density both have 3 significant figures, so our answer must have the same. For this number, 3 sig fig is the hundredth place. The 0 in the thousandth place tells us to leave the 7.
\(4.67 \ g \approx m\)
The mass of the gold sample is approximately 4.67 grams.
The limiting reactant is...
A). The reactant that produces the least amount of product
or B). The reactant that produces the most amount of product
I give Brainliest ! plz no links!
Answers
Answer:
A.) produces the least amount of product.
What is the molar concentration a a 12 % sodium chloride solution (MW 58.5)
Answers
The molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To determine the molar concentration of a 12% sodium chloride solution, we need to convert the given percentage concentration into molarity.
First, we need to understand that the percentage concentration refers to the mass of the solute (sodium chloride) relative to the total mass of the solution.
In this case, a 12% sodium chloride solution means that there are 12 grams of sodium chloride in 100 grams of the solution.
To convert this into molar concentration, we need to consider the molar mass of sodium chloride, which is 58.5 g/mol.
We can start by calculating the number of moles of sodium chloride in 12 grams:
Moles of sodium chloride = mass of sodium chloride / molar mass of sodium chloride
Moles of sodium chloride = 12 g / 58.5 g/mol = 0.205 moles
Next, we calculate the volume of the solution in liters using the density of the solution. Since the density is not provided, we assume a density of 1 g/mL for simplicity:
Volume of solution = mass of solution / density
Volume of solution = 100 g / 1 g/mL = 100 mL = 0.1 L
Finally, we calculate the molar concentration (Molarity) by dividing the number of moles by the volume in liters:
Molar concentration = moles of solute / volume of solution
Molar concentration = 0.205 moles / 0.1 L = 2.05 M
Therefore, the molar concentration of a 12% sodium chloride solution is approximately 2.05 M.
To learn more about molarity click here: brainly.com/question/31545539
#SPJ11
What type of elements are found in each group?
Answers
Group 1 of the periodic table consists of hydrogen and the alkali metals. ...
Group 2 consists of the alkaline Earth metals. ...
Groups 3–12 contain transition metals. ...
Groups 13–16 each contain at least one metalloid. ...
Group 17 contains halogens. ...
Group 18 consists of noble gases.
Answer:
Groups are numbered 1–18 from left to right. The elements in group 1 are known as the alkali metals; those in group 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
HOPE IT'S HELP
FOLLØW ME PLEASE THANKS IN ADVANCE
Heart my answer if it's helpful
BRAINLIEST MY ANSWER IF you want
in a nucleic acid, adjacent nucleotides are bound to each other in what way?
Answers
The adjacent nucleotides are bound to each other through a phosphodiester bond in a nucleic acid.
What is nucleic acid?Nucleic acid is a biopolymer made up of nucleotide monomers that make up nucleic acid chains. The nucleotide's three components are a five-carbon sugar, a phosphate group, and a nitrogenous base. Nucleic acids are present in all living cells, including viruses and bacteria, and they play a critical role in storing, transmitting, and expressing genetic information. RNA and DNA are two types of nucleic acids.
The phosphate group in one nucleotide forms a phosphodiester bond with the hydroxyl group on the sugar molecule of the next nucleotide in line in nucleic acids. This reaction is carried out by removing a molecule of water, resulting in a strong covalent bond between two nucleotides. These bonds make up the sugar-phosphate backbone of a nucleic acid chain, which is fundamental to its structure.
Learn more about Nucleic acid: https://brainly.com/question/17701344
#SPJ11
Copy the sentences below. Correcting the five mistakes.
In a displacement reaction, a less reactive metal pushes out a more reactive metal from its compound. For example, iron displaces aluminium from aluminium oxide.
Answers
Answer:
In a displacement reaction, a more reactive metal pushes out a less reactive metal from its compound. For example, aluminium displaces iron from iron oxide.
Explanation:
Because aluminium is more reactive than iron, it displaces iron from iron(III) oxide. The aluminium removes oxygen from the iron(III) oxide: iron is reduced.
...
Hope this answer can help you. Have a nice day!
In a displacement reaction, a more reactive metal pushes out a less reactive metal from its compound. For example, aluminium displaces iron from iron oxide.
What is a compound?Compound is defined as a chemical substance made up of identical molecules containing atoms from more than one type of chemical element.
Molecule consisting atoms of only one element is not called compound.It is transformed into new substances during chemical reactions. There are four major types of compounds depending on chemical bonding present in them.They are:
1)Molecular compounds where in atoms are joined by covalent bonds.
2) ionic compounds where atoms are joined by ionic bond.
3)Inter-metallic compounds where atoms are held by metallic bonds
4) co-ordination complexes where atoms are held by co-ordinate bonds.
They have a unique chemical structure held together by chemical bonds Compounds have different properties as those of elements because when a compound is formed the properties of the substance are totally altered.
Learn more about compound,here:
https://brainly.com/question/13516179
#SPJ2
It consists of two lobes that contain pollen sacs and pollen grains.
Answers
Answer:
Assuming you need the names :)
Explanation:
It's dithecous anther