Which of the following models best demonstrates the law of conservation of mass??
(LOOK AT THE PICTURES)!!

Answers
Answer:
i think its D
Explanation:
The law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass cannot change, so quantity can neither be added nor be removed.
Taking into account the law of conservation of mass,
The Law of Conservation of Matter is also called the law of conservation of mass or the Law of Lomonósov-Lavoisier. This law postulates that "The mass is not created or destroyed, only transformed."
This means that the reagents interact with each other and form new products with different physical and chemical properties than those of the reagents. And this is because the atoms of the substances are ordered differently.
But the quantities of the masses involved in a given reaction must be constant throughout it, that is, they will not have changed in their proportions when the reaction ends. Then the mass before the chemical reaction is equal to the mass after the reaction.
The exception to the rule is nuclear reactions, in which it is possible to convert mass into energy and vice versa.
Finally, the correct answer is option A. because the atoms of the substances are ordered differently, this is the reagents interact with each other and form new products.
Learn more about law of conservation of mass:
https://brainly.com/question/20081187?referrer=searchResultshttps://brainly.com/question/23986198https://brainly.com/question/13410855Related Questions
What is the volume of 1.25 moles of a nickel sample if the density of a nickel is 8.90g/ml
Answers
Answer:
8.24 mL
Explanation:
Density can be defined as mass per unit volume, according to the relationship shown in the attached image. Moles (a unit of measurement for small entities) can also be related to mass. Let's look at two formulas which would be useful for answering this question.
\(\boxed{\text{Density}=\frac{\text{Mass}}{\text{Volume}} }\)
\(\boxed{\text{Mole}\times\text{Mr}=\text{Mass (in grams)}}\) , where Mr stands for relative molecular mass
From the periodic table the Mr of nickel is 58.7.
Multiply the number of moles by the Mr:
Mass of Ni
= 1.25(58.7)
= 73.375 g
Calculate the volume of Ni:
Volume
= mass ÷density
= 73.375 ÷8.90
= 8.24 ml (3 s.f.)
To learn more about density, check out: https://brainly.com/question/15278418

the poh of a solution is equal to: select the correct answer below: −log[h3o ] log[h3o ] –log[oh−] log[oh−]
Answers
The poh of a solution is equal to is -log[H3O+]. The correct option is a
pH is a measure of the concentration of hydrogen ions (H+) in a solution. The concentration of H+ in an aqueous solution can be expressed using the concentration of hydronium ions (H3O+). pH is defined as the negative logarithm (base 10) of the H3O+ concentration. Therefore, pH = -log[H3O+].
To determine the pH of a solution, you need to know the concentration of H3O+ in the solution and then use the pH formula (-log[H3O+]) to calculate it. pH is the measure of hydrogen ion concentration in a solution and is expressed as the negative logarithm of the concentration of H3O+.
To know more about hydronium ions visit:
brainly.com/question/14619642
#SPJ11
250 kJ of energy are removed from a 4.00 x 10^2 g sample of water at 60°C. Will the
sample of water completely freeze? Explain
Answers
The mass of the sample is 400 g and only 748 g will freeze, it can be concluded that the entire sample of water will not completely freeze.
It is necessary to determine the amount of heat necessary for the water to completely freeze. The heat required to solidify water depends on its specific heat capacity, c, which is the amount of heat energy that is needed to raise the temperature of 1 gram of a substance by 1 degree Celsius, and its heat of fusion, ΔHfus, which is the amount of heat energy that is required to change 1 gram of a substance from a liquid to a solid state. To determine if a sample of water will completely freeze after a given amount of energy is removed, it is important to compare the energy that is removed from the water with the energy that is required for it to freeze.
The equation for the heat change that occurs when a substance changes temperature is Q = mcΔT, where Q is the amount of heat energy that is transferred, m is the mass of the substance, c is its specific heat capacity, and ΔT is the change in temperature. For water, c = 4.18 J/g°C. To convert this to kJ/g°C, divide by 1000:
c = 4.18 J/g°C ÷ 1000 = 0.00418 kJ/g°C
Thus, the heat energy required to change the temperature of 1 gram of water by 1 degree Celsius is 0.00418 kJ.
The energy that is required to solidify water is given by Q = mΔHfus, where m is the mass of the substance and ΔHfus is its heat of fusion. For water, ΔHfus = 6.01 kJ/mol. To convert this to kJ/g, divide by the molar mass of water:
ΔHfus = 6.01 kJ/mol ÷ 18.02 g/mol = 0.334 kJ/g
Thus, the heat energy required to solidify 1 gram of water is 0.334 kJ.
In this case, 250 kJ of energy are removed from a 4.00 x 102 g sample of water at 60°C.
The change in temperature that will occur due to the removal of energy can be determined using the equation Q = mcΔT. Rearranging this equation to solve for ΔT gives:
ΔT = Q ÷ mc
ΔT = 250 kJ ÷ (4.00 x 102 g) x (0.00418 kJ/g°C)
ΔT ≈ 14.9°C
Thus, the temperature of the water will decrease from 60°C to 45.1°C.
The mass of the water that will freeze can be determined using the equation Q = mΔHfus. Rearranging this equation to solve for m gives:
m = Q ÷ ΔHfus
m = 250 kJ ÷ 0.334 kJ/g
m ≈ 748 g
Thus, 748 grams of water will freeze.
To know more about freeze visit:-
https://brainly.com/question/26230908
#SPJ11
16 G of oxygen at 15° above 18 how many liters of oxygen is in a container
Answers
Answer:
To determine the volume of 16 g of oxygen at 15°C and 1 atm pressure, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure in atm, V is the volume in liters, n is the number of moles, R is the gas constant (0.0821 L atm/mol K), and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
T = 15°C + 273.15 = 288.15 K
Next, we need to calculate the number of moles of oxygen:
n = m/M
Where m is the mass of oxygen (16 g) and M is the molar mass of oxygen (32 g/mol).
n = 16 g / 32 g/mol = 0.5 mol
Now we can rearrange the ideal gas law equation to solve for V:
V = nRT/P
V = (0.5 mol)(0.0821 L atm/mol K)(288.15 K) / 1 atm = 11.3 L
Therefore, there are 11.3 liters of oxygen in the container.
Explanation:
This same chemistry student has a weight of 155 lbs. What is the student’s weight in
grams? (16oz=1lb, 1 oz = 28.34 g)
Answers
Answer:
the same chemistry student has a weight of 155lbs what is the student weight in grams? (16 oz= 1 lb, 1 oz= 28.34g)
Explanation:1 lb = 16oz, so multiply your pounds by 16 to get you ounces of the student, then multiply by 28.34 to get grams
155 X 16 X 28.34 = 70283.2
Answer:
The student weighs 70306.8 grams.
How do the coefficients in a balanced equation compare quantities of two
substances?
A. The coefficient of the reactant tells how many product molecules
will form,
B. The ratio of the coefficients equals the ratio of the masses of the
substances,
C. The sum of the coefficients of the reactants equals the sum of the
products,
D. The ratio of the substances' coefficients equals the ratio of their
numbers of moles,
Answers
Answer:
D. The ratio of the substances coefficients equals the ratio of their numbers of moles.
How many molecules are in 2,10 mol CO2?
0 3.79 X 1024 molecules
O 2.53 x 1024 molecules
O 1.26 X 1024 molecules
O 3.49 * 10-24 molecules
Answers
Answer:
the answer is the 3rd option
Explanation:
which is 1.26 x 1024 calculating with avogardos law
Answer:
1.26 X 1024 molecules
Explanation:
I need help on this question

Answers
GUYS I NEED HELP WITH THIS ASSIGNMENT MY LAST ASSIGNMENT FOR THE DAY
1. What are the reactants? What are the products? Use the reaction below (2pts)
Na + Cl2 → NaCl
2. Why do chemical reactions need to be balanced? (2pt)
3. In order to balance the following reaction, I need to add more Chlorine atoms to the product side. Would the highlighted answer be a correct way of adding more Chlorine atoms? Why or why not? (2pt)
Balanced: Na + Cl2 → NaCl2
4. Given the following reactions, what does the coefficient 2 represent for 2KI? (2pt)
Cl2 + 2 KI → 2 KCl + I2
Answers
Answer:
the reactants are na and cl2 the products is the combination nacl --the law of conservation of matter keeps reactions balanced. No you would not be adding more atoms that is what the first combo is. The coefficient 2 stands for 2 atoms of KL
Explanation:
A neutral (net charge
zero) atom of silicon contains:
A
28.09 electrons
B
16 electrons
C
14 electrons
D
38 electrons
Answers
Answer:
C 14
Explanation:
Silicon atom has 14 protons. Given that a positive and a negative neutralize each other you need 14 electrons to neutralize the 14 protons.
Carbon tetrachloride, CCl4 , was once used as a dry cleaning solvent, but is no longer used because it is careinegenic. At 56.4 ∘ C, the vapar pressure of CO 4 is 53.7kPa, and its enthalpy of vaporiatian is 29.82 kJ/mol. Use this infoation to estimate the noal boiling point (in ∘C ) for CCl4 [ill "C
Answers
The normal boiling point of carbon tetrachloride (CCl4) can be estimated using the given information. The estimated boiling point is approximately 76.5 °C.
The enthalpy of vaporization (ΔHvap) is the amount of heat required to convert one mole of a substance from a liquid to a gas at its boiling point. In this case, the enthalpy of vaporization for CCl4 is given as 29.82 kJ/mol.
The vapor pressure of a substance at a particular temperature is the pressure exerted by its vapor in equilibrium with its liquid phase. The vapor pressure of CCl4 at 56.4 °C is given as 53.7 kPa.
The boiling point of a substance is the temperature at which its vapor pressure equals the atmospheric pressure. At the normal boiling point, the vapor pressure is equal to 101.3 kPa.
To estimate the normal boiling point of CCl4, we can set up a proportion using the vapor pressures:
53.7 kPa / 101.3 kPa = x °C / 56.4 °C
Simplifying the equation, we have:
x = (53.7 kPa / 101.3 kPa) * 56.4 °C
x ≈ 29.9 °C
Therefore, the estimated normal boiling point of carbon tetrachloride is approximately 76.5 °C.
Learn more about boiling point
brainly.com/question/29981108
#SPJ11
Colorless hydrogen gas and violet iodine gas are introducted into a sealed vessel and react to form colorless hydrogen iodide. What happens to the concentrations of hydrogen, iodine and hydrogen iodide as equilibrium is established?
Answers
When colorless hydrogen gas and violet iodine gas react to form colorless hydrogen iodide in a sealed vessel, an equilibrium is established. At equilibrium, the concentrations of hydrogen, iodine, and hydrogen iodide reach a constant value. The specific concentrations of each species will depend on the reaction conditions and the equilibrium constant (K) for the reaction.
In this particular reaction, the balanced equation is:
H2(g) + I2(g) ⇌ 2HI(g)
As the reaction proceeds towards equilibrium, the concentrations of the reactants (hydrogen and iodine) will decrease, while the concentration of the product (hydrogen iodide) will increase until the reaction reaches equilibrium.
The exact concentrations of hydrogen, iodine, and hydrogen iodide at equilibrium will depend on factors such as the initial concentrations of the reactants, temperature, and pressure. The equilibrium concentrations will be determined by the equilibrium constant (K), which is specific to the particular reaction.
If the reaction conditions are such that the equilibrium constant (K) for the reaction is large, it indicates that the forward reaction (formation of hydrogen iodide) is favored at equilibrium. In this case, the concentration of hydrogen iodide will be relatively high compared to the concentrations of hydrogen and iodine.
On the other hand, if the equilibrium constant (K) is small, it indicates that the reverse reaction (decomposition of hydrogen iodide) is favored at equilibrium. In this case, the concentration of hydrogen iodide will be relatively low compared to the concentrations of hydrogen and iodine
It's important to note that at equilibrium, the reaction does not stop completely but reaches a dynamic state where the forward and reverse reactions occur at equal rates. This results in a constant concentration of each species.
To summarize, at equilibrium in the reaction between hydrogen and iodine to form hydrogen iodide, the concentrations of hydrogen and iodine will decrease, while the concentration of hydrogen iodide will increase until reaching a constant value determined by the equilibrium constant and reaction conditions.
Learn more about equilibrium Visit : brainly.com/question/517289
#SPJ11
How does oxygen obey the octet rule
Answers
Answer:
oxygen obey the octet rule when reacting to form compounds by adding 2 electrons.
Explanation:
Answer:
See below.
Explanation:
An oxygen atom, in free state has 8 electrons, 2 in the first shell, and 6 in the second. It can only achieve stability if the valency of the valence shell is 0. In order for this, it combines with another atom with the same valency, such as magnesium or another oxygen atom to obey the octet rule. Hope it helps.
Which of the following reactions of alkenes is NOT stereospecific? A Hydrogenation (H2/P1) o B Bromination (Br2 in CH2Cl2) o c Acid-catalyzed hydration (H20/H2504) O D Bromohydrin formation (Br2/H20)
Answers
The following reactions of alkenes is not stereospecific is C. acid-catalyzed hydration reaction (H₂O/H₂SO₄)
Stereospecific reactions occur when the stereochemistry of the reactant is retained in the product. These types of reactions are distinguished by the use of double-headed arrows in reaction mechanisms to demonstrate the conservation of stereochemistry. The following reactions of alkenes stereospecific are hydrogenation (H₂/P₁), bromination (Br₂ in CH₂Cl₂), bromohydrin formation (Br₂/H₂O).
The acid-catalyzed hydration reaction (H₂O/H₂SO₄) of alkenes is not stereospecific because the H and OH atoms can be added to either face of the alkene's double bond. When the reaction occurs, an intermediate carbocation is formed, which is planar. This carbocation can either be attacked by the nucleophile from above or below, resulting in the formation of an equal amount of stereoisomers. Therefore, the correct answer is C. acid-catalyzed hydration reaction (H₂O/H₂SO₄) is not stereospecific.
Learn more about stereospecific at:
https://brainly.com/question/22811170
#SPJ11
Fluorine, chlorine, and iodine are examples of
Answers
Answer:
Halogens
Explanation:
The halogens are a series of non-metal elements from group 17 of the periodic table (formerly VII). The halogens include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Answer:
halogens
Explanation:
just did the test on edg!
Rank the following species by radius, from largest to smallest:
cl−, k , ca2, ar, s2−.
Answers
The rank of the species by radius from largest to smallest: \(S^{2-} > Cl^{-} > Ar > K^{+} > Ca^{2+}\)
All Ions are isoelectronic, which means the elements can have the same number of electrons but, their radii would be different because they have different nuclear charges. Atomic radii can be predicted easily. The atomic radii in the periodic table increase from top to bottom coming from left to right.
The cation with a greater positive charge will have a small radius due to the great attraction of electrons. The Anion with a greater negative charge will have a large radius due to the less attraction of electrons. Therefore, the ion will expand in size due to the net repulsion of the electrons.
To know more about Ranking the species by radius from largest to smallest at:
https://brainly.com/question/19032815
(b) A student states, "When the alcohol sample was at a temperature of 500 K, all the particles
were moving faster than any of the particles were moving at 400 K." Do you agree or disagree
with this student's statement? Justify your answer.
B
I
Submit
514
alel
(c) Using boxes like the ones below, draw three particle diagrams representing the sample of
alcohol cooling in this experiment. The diagrams should represent the sample at 3 minutes, 12
minutes, and 17 minutes.
Sign out
09:50
Answers
The kinetic energy of particles depends on temperature. As temperature increases, the kinetic energy of particles increases.
Temperature is defined as a measure of the average kinetic energy of the molecules of a substance. The higher the temperature of a substance the greater its kinetic energy.
From the foregoing, when the temperature is increased from 400K to 500K, greater kinetic energy is imparted to the molecules of the substance hence they move faster according to the kinetic theory of matter.
Learn more: https://brainly.com/question/10079361
Question (a)Calculate the empirical formula of a compound that has the following composition by mass. 29.0% Na, 40.5% S, O=30.4%
Answers
The first step to answer this question is to assume that the given percents are masses.
Then, convert these masses to moles using the corresponding molar mass:
\(\begin{gathered} 29.0gNa\cdot\frac{1molNa}{22.98gNa}=1.26molNa \\ 40.5gS\cdot\frac{1molS}{32.065gS}=1.26molS \\ 30.4gO\cdot\frac{1molO}{15.999gO}=1.9molO \end{gathered}\)Divide each result by the least result (1.26mol):
\(\begin{gathered} \frac{1.26mol}{1.26mol}=1 \\ \frac{1.26mol}{1.26mol}=1 \\ \frac{1.9mol}{1.26mol}=1.5 \end{gathered}\)Since not all of these are whole numbers, we have to multiply them times 2:
\(\begin{gathered} 1\cdot2=2 \\ 1\cdot2=2 \\ 1.5\cdot2=3 \end{gathered}\)These numbers are the subscripts of each element in the empirical formula.
It means that the empirical formula of this compound is:
\(Na_2S_2O_3\)First, state your claim about how the Mesosaurus fossils got separated. Then, use evidence to support your claim. For each piece of evidence you use, explain how the evidence supports your claim. Be sure to include the words from the Word Bank!
Answers
Claim: The separation of Mesosaurus fossils can be explained by the continental drift hypothesis. Evidence: Fossil Distribution, Geological Similarities, Matching Climate and Habitat. The distribution of Mesosaurus fossils across South America and Africa, the geological similarities between these regions, and the matching climate and habitat conditions all support the claim that the separation of Mesosaurus populations can be explained by the process of continental drift.
Claim: The separation of Mesosaurus fossils can be explained by the continental drift hypothesis.
Evidence:
Fossil Distribution: Mesosaurus fossils are found in both South America and Africa. This distribution aligns with the hypothesis of continental drift, which suggests that these continents were once connected and later separated. The similarity in fossil remains on different continents supports the claim that Mesosaurus populations were separated when the continents drifted apart.
Geological Similarities: The geological formations and sedimentary layers in which Mesosaurus fossils are found in South America and Africa display remarkable similarities. This similarity implies that these regions were once part of the same landmass and were subsequently separated. The matching geological features provide further evidence for the separation of Mesosaurus populations due to continental drift.
Matching Climate and Habitat: Mesosaurus fossils indicate that the species was adapted to a freshwater environment. The presence of similar freshwater environments in both South America and Africa further supports the claim that Mesosaurus populations were separated when the continents drifted apart. The matching climate and habitat conditions provide evidence that supports the idea of geographic isolation and subsequent speciation.
In conclusion, the distribution of Mesosaurus fossils across South America and Africa, the geological similarities between these regions, and the matching climate and habitat conditions all support the claim that the separation of Mesosaurus populations can be explained by the process of continental drift.
For more question on climate
https://brainly.com/question/29617595
#SPJ8
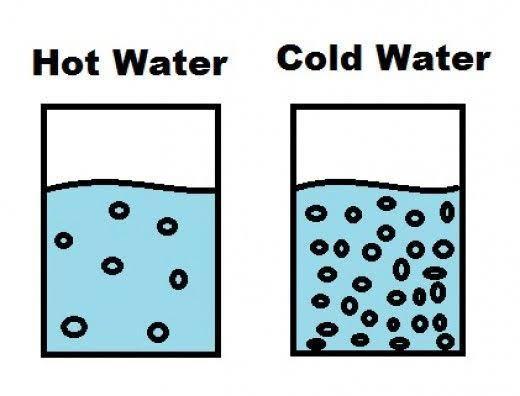
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
Which type of reaction always requires oxygen to be a reactant?
Answers
Answer:
I think it's a combustion reaction
Explanation:
how many Oxygen atoms are in 3.15 mol of SnO2
Answers
Answer: 3.7926x1024 O atoms
As continents come together to form a supercontinent,
a. the ocean that jad separated them disappears
b. a giant ocean forms on the outside of the supercontinent
c. mountain ranges rise where continental plates converge
d. all of these
Answers
Calcium hydroxide, Ca(OH)2, is an ionic compound with a solubility product constant, Ksp, of 6.5×10–6. Calculate the solubility of this compound in pure water.
Answers
Answer: The solubility of this compound in pure water is 0.012 M
Explanation:
Solubility product is defined as the equilibrium constant in which a solid ionic compound is dissolved to produce its ions in solution. It is represented as \(K_{sp}\)
The equation for the ionization of the is given as:
\(Ca(OH)_2\rightarrow Ca^{2+}+2OH^-\)
By stoichiometry of the reaction:
1 mole of \(Ca(OH)_2\) gives 1 mole of \(Ca^{2+}\) and 2 mole of \(OH^-\)
When the solubility of \(Ca(OH)_2\) is S moles/liter, then the solubility of \(Ca^{2+}\) will be S moles\liter and solubility of \(OH^-\) will be 2S moles/liter.
\(K_{sp}=[Ca^{2+}][OH^{-}]^2\)
\(6.5\times 10^{-6}=[S][2S]^2\)
\(S=0.012M\)
Thus solubility of this compound in pure water is 0.012 M
when the foil is negatively charged will all of the foil still be made up of aluminum atoms
Answers
Answer:
Yes.
Explanation:
Electric charge cannot change the elemental composition of a substance.
The application of charge to the aluminium foil does not change the composition of the foil and hence the foil will still be made up of aluminium atoms.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/14214017
#SPJ2
Fill in the blank
Potential energy is energy an object has due to its ____
or condition
Answers
Answer :position
Explanation:
Potential energy is the energy stored in an object due to its position
What point defects are possible for Al2O3 as an impurity in MgO? How many Al^3+ ions must be added to form each of these defects?
Answers
The possible point defects that can be formed are Frenkel Defect and Schottky Defect. To form one Frenkel defect, one Al³⁺ ion is needed. To form one Schottky defect, one Al³⁺ ion and one O²⁻ ion are required.
When Al₂O₃ is used as an impurity in MgO, it can introduce various point defects into the crystal structure. The possible point defects that can be formed are
Frenkel Defect
In a Frenkel defect, an ion (usually a cation) is displaced from its lattice site into an interstitial position. In this case, an Al³⁺ ion from Al₂O₃ can occupy an interstitial position in the MgO lattice. To form one Frenkel defect, only one Al³⁺ ion is required.
Schottky Defect
In a Schottky defect, an equal number of cations and anions are missing from their lattice positions, creating vacancies. Here, both Al³⁺ ions and O²⁻ ions from Al₂O₃ can create vacancies in the MgO lattice. To form one Schottky defect, one Al³⁺ ion and one O²⁻ ion are required.
It's important to note that the exact number of Al³⁺ ions required to form these defects depends on the stoichiometry and the charge balance of the system. The examples given above assume a 1:1 stoichiometry between MgO and Al₂O₃.
Therefore, to form one Frenkel defect, one Al³⁺ ion is needed. To form one Schottky defect, one Al³⁺ ion and one O²⁻ ion are required.
To know more about Frenkel Defect here
https://brainly.com/question/2004968
#SPJ4
I.Molten Nacl conducts electricity due to the presence a
a-free electrons
b. free ions
c. Free molecules .
d. Atoms of sodiium and chloride.
Answers
Answer:
Hello! Your answer would be BELOW
Explanation:
So a molten salt powers electricity. Hence, molten sodium chloride conducts electricity due to the presence of free ions. So, the correct answer is option D. Atoms of sodium and chloride.
Hope I helped! Hope you make an 100%
-Amelia♥
12. Switch out the fluorescent bulb with the regular bulb and observe the energy output.
What do you notice about the difference in the energy and output of these two bulbs?
Answers
The fluorescent bulb has a better energy output than the regular bulb.
What is the difference between the energy output of the fluorescent and the regular bulb?The main difference between the energy output of a fluorescent bulb and a regular (incandescent) bulb is their efficiency. Fluorescent bulbs are much more energy-efficient than incandescent bulbs because they convert a much higher proportion of the energy they consume into visible light, rather than heat.
Incandescent bulbs work by heating a filament inside the bulb until it glows, emitting light. However, this process is very inefficient, with most of the energy consumed being lost as heat. In contrast, fluorescent bulbs use a small amount of energy to excite a gas inside the bulb, which then emits ultraviolet (UV) light.
Learn more about bulbs:https://brainly.com/question/15932311
#SPJ1
Rank the following nitrogen compounds in order of decreasing oxidation number for nitrogen.
NO3^- NO2^- NH3 N2 NO2 NO
Answers
The oxidation number of an atom is the number of valence electrons subtracted or added to the total number of electrons in the atom. Oxidation number is an important concept in chemistry, especially in redox reactions. Nitrogen is a Group 15 element and has five valence electrons.
Nitrogen in the compounds can have oxidation states ranging from -3 to +5. As nitrogen has five valence electrons, nitrogen-containing compounds can have a range of oxidation states.
The oxidation state of nitrogen can be calculated by following these rules:
Oxidation state of nitrogen in NH3: In NH3, each hydrogen atom has an oxidation state of +1, which means the nitrogen atom must have an oxidation state of -3 to balance the total charge to zero. Oxidation state of nitrogen in NO: In NO, the oxygen atom has an oxidation state of -2, which means that the nitrogen atom has an oxidation state of +2 to balance the total charge to zero. Oxidation state of nitrogen in NO2:In NO2, each oxygen atom has an oxidation state of -2, which means that the nitrogen atom has an oxidation state of +4 to balance the total charge to zero.
Oxidation state of nitrogen in NO3-:In NO3-, each oxygen atom has an oxidation state of -2, which means that the nitrogen atom has an oxidation state of +5 to balance the total charge to zero.
Oxidation state of nitrogen in N2:N2 is a covalent molecule, which means that each nitrogen atom has an oxidation state of 0.
Rank the following nitrogen compounds in order of decreasing oxidation number for nitrogen:
NO3- > NO2- > NO2 > NH3 > N2 > NO.
So, the order of nitrogen compounds in decreasing order of oxidation number for nitrogen is: NO3- > NO2- > NO2 > NH3 > N2 > NO.
To know more about oxidation visit-
https://brainly.com/question/13182308
#SPJ11