Answers
Answer:
C
Explanation:
edg
Answer:
C
Explanation:
got it right on Edge
Related Questions
At 20C what is the molar mass of a gas with a denisty of 1.02g/L at 2.13atm
Answers
The molar mass of a gas with a density of 1.02 g/L at 2.13 atm and a temperature of 20°C is 47.9 g/mol.The molar mass of an element or compound is the mass of one mole of that substance. A mole is the SI unit for the amount of a substance.
It's defined as the amount of a substance that contains the same number of entities as there are atoms in 12 grams of carbon-12.Molar mass (M) = mass (m) ÷ amount of substance (n)So, M = m/n
Where m is the mass in grams and n is the number of moles. The unit of molar mass is grams per mole (g/mol).
The ideal gas law is used to calculate the molar mass of a gas. The ideal gas law is:P V = n R T,Where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
Convert the density to grams per liter: 1.02 g/L.
The density is mass/volume, thus 1.02 g/L means that 1 liter of the gas weighs 1.02 g.
This means that 1 mole of gas will occupy 22.4 L (at standard temperature and pressure, STP).Calculate the number of moles of gas using PV = nRT.P = 2.13 atmV = 22.4 L (at STP)R = 0.0821 L·atm/K·molT = 273.15 K + 20 K = 293.15 K
Thus, n = PV/RT = (2.13 atm × 22.4 L)/(0.0821 L·atm/K·mol × 293.15 K) = 0.973 mol
Calculate the molar mass (M) using M = m/n.m = density × volume = 1.02 g/L × 22.4 L = 22.848 gM = m/n = 22.848 g/0.973 mol = 23.5 g/mol Convert to units of grams per mole: 23.5 g/mol
The molar mass of a gas with a density of 1.02 g/L at 2.13 atm and a temperature of 20°C is 47.9 g/mol.
For more question on compound
https://brainly.com/question/12651906
#SPJ8
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Arsenic(III) sulfide sublimes readily, even below its melting point of 320 °C. The molecules of the vapor phase are found to effuse through a tiny hole at 0.28 times the rate of effusion of Ar atoms under the same conditions of temperature and pressure. What is the molecular formula of arsenic(III) sulfide in the gas phase?
Answers
The molecular formula : As₄S₆
Further explanationGiven
Rate of effusion of arsenic(III) sulfide = 0.28 times the rate of effusion of Ar atoms
Required
The molecular formula
Solution
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
\(\rm \dfrac{r_1}{r_2}=\sqrt{\dfrac{M_2}{M_1} }\)
or
\(\rm M_1\times r_1^2=M_2\times r_2^2\)
Input the value :
1 = Arsenic(III) sulfide
2 = Ar
MM Ar = 40 g/mol
0.28 = √(40/M₁)
M₁=40 : 0.28²
M₁=510 g/mol
The empirical formula of arsenic(III) sulfide = As₂S₃
(Empirical formula)n = molecular formula
( As₂S₃)n = 510 g/mol
(246.02 g/mol)n = 510 g/mol
n = 2
So the molecular formula : As₄S₆
Define inference and explain how it is used to form conclusions.
Answers
Answer:
an inference is an educated answer based on a piece of information you have been given
Explanation:
e.g. they had pigtails in their long blonde hair.
from this we can infer that I am speaking about a girl
These three gases, Helium, Neon, and Argon all have one property in common because they are gases at room temperature. That is they:

Answers
They have no definite shape and no definite volume because they are gases.
How gases have no definite and volume?Gases have no definite shape and no definite volume because they are present in free state. Those substances that are close together and held tightly have definite shape and volume.
So we can conclude that they have no definite shape and no definite volume because they are gases.
Learn more about gas here: https://brainly.com/question/25736513
#SPJ1
Which one of the following salts is least soluble in water?
1. Na2SO4
2.CaBr2
3. LiCl
4. RbI
5. PbSO4
Answers
Karl went to the doctor complaining of a sore throat. The doctor performed
a throat culture and determined that antibiotics were not necessary. The
doctor told her to drink tea, take ibuprofen, and rest. Which type of microorganism most likely caused Karl's sore throat?
a) bacteria
b) virus
c) parasite
d) fungi
Answers
Answer:
A
Explanation:
bc
what is the full answer with steps
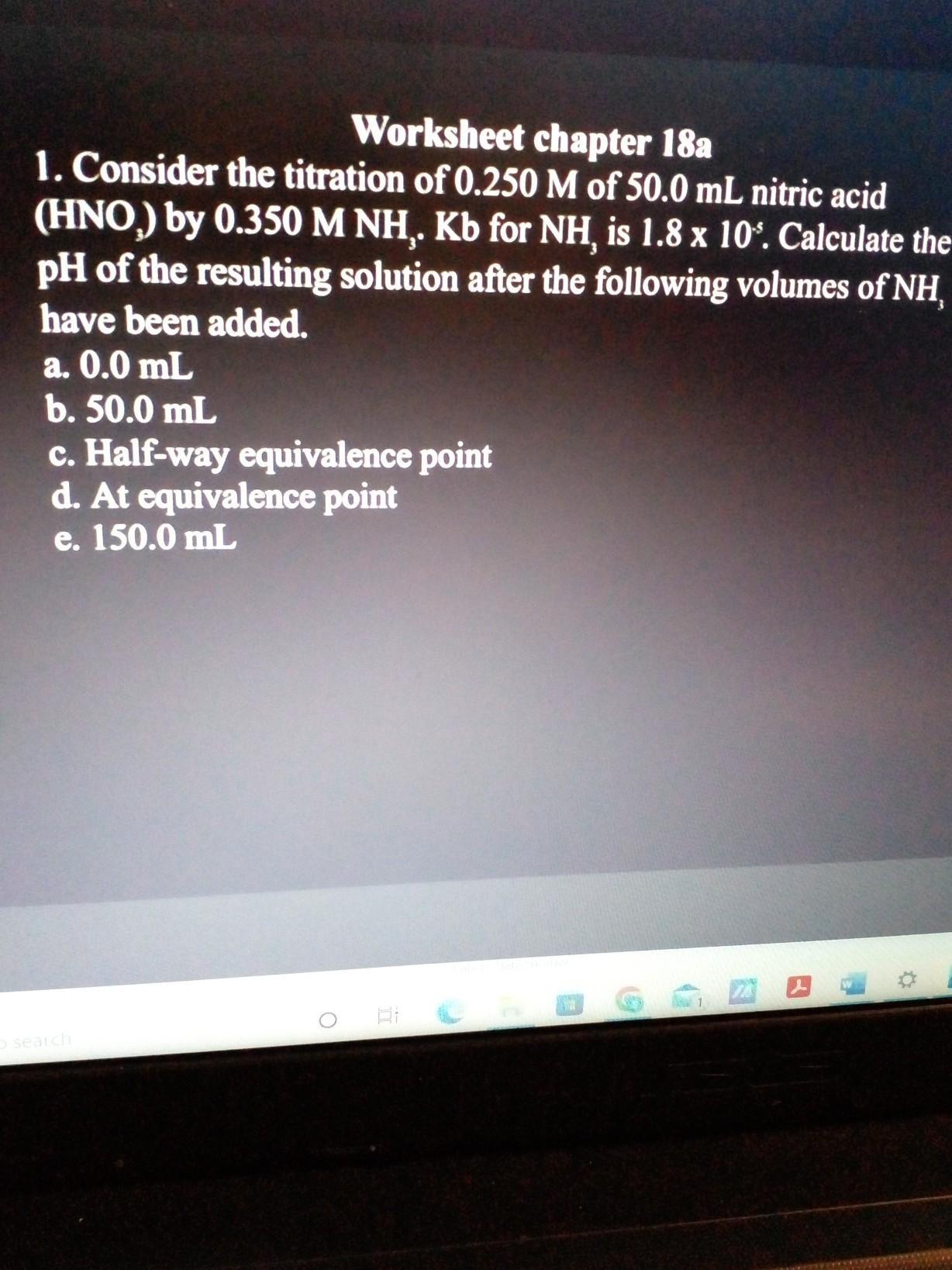
Answers
After adding the next amounts of NH, the final solution's pH was 4.98. Strong acid plus weak base equals an acidic pH, which is supported by this.
If you'll note, NH3 and HNO3 have the same volume and concentration, while HNO3 only contains one H.
As a result, you should persuade yourself that these react exactly as they should because their mol quantities are equivalent.
Make use of the following equation to determine pH:
pH = -log [H+]
The pH formula can be used to determine the pH of a chemical solution and how acidic or basic it is: pH equals -log10[H 3 O +]. Anything below 7 is acidic, and everything over 7 is basic. Learn how to calculate the pH of any chemical solution using the procedures listed below.
To Identify pH true meaning:
The concentration of hydrogen ions in a solution is determined by the pH scale. A solution having a lot of hydrogen ions in it is acidic. Normally, the pH scale is displayed from 0 to 14. The solution is more straightforward the larger the number.
To learn more about Calculating pH, visit
https://brainly.com/question/13008689
#SPJ13
Please someone answer this asap

Answers
Answer:
b or c but I would just pick c
Answer:
C
Explanation:
Sun is an energy source not a matter.
A 1.00 liter solution contains 0.42 moles nitrous acid and 0.32 moles sodium nitrite .
If 0.16 moles of nitric acid are added to this system, indicate whether the following statements are true or false.
(Assume that the volume does not change upon the addition of nitric acid.)
A. The number of moles of HNO2 will decrease.
B. The number of moles of NO2- will remain the same.
C. The equilibrium concentration of H3O+ will increase.
D. The pH will decrease.
E. The ratio of [HNO2] / [NO2-] will increase
Answers
Answer:
E. The ratio of [HNO2] / [NO2-] will increase
D. The pH will decrease.
Explanation:
Nitrous acid ( HNO₂ ) is a weak acid and NaNO₂ is its salt . The mixture makes a buffer solution .
pH = pka + log [ salt] / [ Acid ]
= 3.4 + log .32 / .42
= 3.4 - .118
= 3.282 .
Now .16 moles of nitric acid is added which will react with salt to form acid
HNO₃ + NaNO₂ = HNO₂ + NaNO₃
concentration of nitrous acid will be increased and concentration of sodium nitrite ( salt will decrease )
concentration of nitrous acid = .42 + .16 = .58 M
concentration of salt = .32 - .16 = .16 M
ratio of [HNO₂ ] / NO₂⁻]
= .42 / .32 = 1.3125
ratio of [HNO₂ ] / NO₂⁻] after reaction
= .42 + .16 / .32 - .16
= 58 / 16
= 3.625 .
ratio will increase.
Option E is the answer .
pH after reaction
= 3.4 + log .16 / .58
= 2.84
pH will decrease.
What is Centripetal force?
A. A force that acts on a body moving in a circular path
B. A gravitational force
C. A petal that turns
Answers
A. A force that acts on a body moving in a circular path
A student is demonstrated a transformation of kinetic and potential energy in a system using the pendulum shown below at which point is the system does the pendulum bob demonstrate minimum potential energy 
Answers
The potential energy of the pendulum can be modeled off of the basic equation PE = mgh.
How do you find the potential and kinetic energy of a pendulum?where h is the height and g is the acceleration brought on by gravity. This equation is frequently used to simulate items falling freely.The pendulum is not falling freely; instead, it is restrained by the rod or string.
As a result, we must express the height in terms of the angle and the pendulum's length L. Therefore, h = L(1 - COS) The formula for kinetic energy is KE= 12mv2, where m is the pendulum's mass and v is its speed. The pendulum is temporarily immobile when it is at its highest point (Point A).
There is no kinetic energy in the pendulum; all of its energy is gravitational potential energy. If friction and other non-conservative factors are ignored, we see that in a straightforward.
To learn more about potential and kinetic energy refer to :
https://brainly.com/question/18963960
#SPJ1
Edmentum
What do these circuits all have in common?
A.
They all contain switches.
B.
They are all AC.
C.
They are all DC.
D.
They all contain resistors.
Answers
Answer:
They all contain switches
Answer: D. they all contain resonators.
Explanation:
PLZ HELP I NEED THIS RN!
when the balls reach the bottom of the ramp, which statement will describe their energy?
A. they will have no kinetic energy
B. they will have 100% potential energy
C. they will have 100% kinetic energy
C. they will have different amounts of potential energy
Answers
Answer:
they will have 100% kinetic energy
Explanation:
all the potential energy has been used when it is at the bottom of the ramp
are any substance made up of matter and can be natural or man-made.
Answers
Answer:
Yes.
Explanation:
Substances are made up of matter or matter are made up of tiny molecules or atoms that occur naturally or some are synthetic or man made.
All matter are made up of substances called elements and each elements have its own physical and chemical properties and cannot be broken easily by ordinary chemical reactions.
Of all the 118 elements only 92 occur naturally and 26 are man made or synthetic which are made in the laboratories.
Which is an example of genetic engineering?
A.
humans’ ability to digest cow milk
B.
some bacteria’s resistance to antibiotics
C.
golden rice enriched with vitamin A
D.
lizards with longer legs for surviving floods
Answers
Answer:
C. Golden rice enriched with vitamin A
Mr. Auric Goldfinger, criminal mastermind, intends to smuggle several tons of gold across international borders by disguising it as lumps of iron ore. He commands his engineer minions to form the gold into little spheres with a diameter of exactly and paint them black. However, his chief engineer points out that customs officials will surely notice the unusual weight of the "iron ore" if the balls are made of solid gold (density ). He suggests forming the gold into hollow balls instead, so that the fake "iron ore" has the same density as real iron ore. Calculate the required thickness of the walls of each hollow lump of "iron ore."
Answers
The given question is not complete, the complete question is:
Solving applied density problems Mr. Auric Goldfinger, criminal mastermind, intends to smuggle several tons of gold across International borders by disguising it as lumps of iron ore. He commands his engineer minions to form the gold into little spheres with a diameter of exactly 6 cm and paint them black However, his chief engineer points out that customs officials will surely notice the unusual weight of the "iron ore" if the balls are made of solid gold (density 19.3 g/cm'). He suggests forming the gold into hollow balls Instead (see sketch at right), so that the fake "Iron ore" has the same density as real iron ore (5.15 g/cm'). One of the balls of fake iron ore," sliced in half Calculate the required thickness of the walls of each hollow lump of iron ore. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
Answer:
The correct answer is 0.29 cm.
Explanation:
To produce fake iron balls that is, formed of gold there is a need to make sure that the mass of the iron ball should be equivalent to the mass of the fake ball.
To calculate the volume of iron ball with the help of the given diameter, the formula to be used is 4/3πr³. The diameter of the spheres mentioned in the given question is 6 cm, so the radius will be 6/2= 3 cm.
Now, the volume of the iron ball would be = 4/3 × 3.14 × 3³ = 113.04 cm³
To determine the mass of the iron ball, the formula to be used is volume * density. Now putting the values we get,
113.04 × 5.15 g/cm³ = 582.156 grams (The density of the iron ore is 5.15 g/cm³)
Now, the mass of the gold ball should also be equal to 582.156 g. The density of the solid gold is 19.3 g/cm³, therefore, the volume of the gold ball by using the above formula would be,
mass of gold ball = Volume × density
Volume = mass of gold ball / density
Volume = 582.156 g / 19.3 g/cm³
= 30.1635 cm³
Thus, the volume of the hollow sphere would be 30.1635 cm³ with the outer radius as 3 cm, now there is a need to find the inner radius.
Volume of hollow ball = 4/3π [R³ -r³]
30.1635 cm^3 = 4/3 pie [3³ = r³]
30.1635 × 3/4 × 3.14 = 27-r³
7.2046 = 27-r³
r³ = 19.7954
r = 2.7051 cm
Hence, the thickness would be outer radius - inner radius = 3-2.7051 = 0.2949 cm or 0.29 cm.
Explain the difference between wavelength and frequency
Answers
Answer:
Wavelength (typically measured in nanometers) is the distance between two points in a wave.Frequency (typically measured in Hertz) is the number of waves in a specific time . Frequency and wavelength have both direct and inverse relationships. The crucial difference between frequency and wavelength is that frequency shows the total number of wave oscillations in a given time. As against wavelength specifies the distance between two specific points of a wave.
Explanation:
Frequency is how often something changes per second be it amplitude of a voltage on a wire or be it the bobbing back and forth of a bobblehead. Frequency is how often something moves up and down in a second. If a bobble head moves forward and backward in one second then it has a bobbling frequency of 1 Hertz (Hz). The unit of frequency is Hertz (Hz) or # of cycles or oscillations per second. A wavelength is measured in distance like meters (m). For photons or light or radiowaves the equation is wavelength=speed of light/frequency.
Answer:
Wavelength (typically measured in nanometers) is the distance between two points in a wave, while frequency is how often something changes per second be it amplitude of a voltage on a wire or be it the bobbing back and forth of a bobblehead.
A sample of an ideal gas has a volume of 2.35L at 2.90x10^2 K and 1.09 atm. Calculate the pressure when the volume is 1.59L and the temperature is 306K
Answers
P1V1/T1=P2V2/T2
Where P1=1.09atm
V1=2.35L
T1=2.90x10^2K
P2=?
V2=1.59L
T2=306K
Solution
1.09atmx2.35L/2.90x10^2K=P2x1.59L/306K
P2=1.09atmx2.35Lx306K/1.59Lx290K
P2=1.09atmx2.35x306/1.59x290
P2=783.819atm/461.1
P2=1.6998atm approximately to 1 decimal =1.7atm.
2H2O -> 2H2 + O2
How many moles of Hy are produced from 72.16 g of H2O?
Answers
Answer:
4moles
Explanation:
2moles produces 2moles of H2
then 4moles (72g) produces 8 grams H2
What best explains whether bromine (Br) or neon (Ne) is more likely to form a covalent bond?
On left, a purple circle labeled Br surrounded by 4 concentric circles. The inner circle has 2 small green spheres. The second circle has 8 small green spheres. The third circle has 18 small green spheres. The fourth circle has 5 small green spheres. On right, a purple circle labeled Ne surrounded by 3 concentric circles. The inner circle has 2 small green spheres. The middle circle has 8 small green spheres. The outer circle has 8 small green spheres.
Bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule.
Bromine forms covalent bonds because it has many electron shells, but neon has only two electron shells and is tightly bound to its electrons.
Neon forms covalent bonds because it can share its valence electrons, but bromine has seven valence electrons and can gain only one more electron.
Neon forms covalent bonds because it has only two electron shells, but bromine has many electron shells and will lose electrons in order to fulfill the octet rule.
Mark this and return
Answers
Answer:
Bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. Neutral atoms coming together to share electrons.
Hoped this helped
Answer:
the answer is A btw
Explanation:
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
What is the number of molecules of NO, which contains 16 gm of oxygen. 14
Answers
To find the number of molecules of NO that contains 16 g of oxygen, we need to first calculate the number of moles of oxygen in 16 g of oxygen. Using the atomic weight of oxygen (16 g/mol), we can calculate:
moles of O = mass of O / atomic weight of O = 16 g / 16 g/mol = 1 mol
Next, we need to determine the number of moles of NO that contains 1 mol of oxygen. From the molecular formula of NO, we can see that 1 mol of NO contains 1 mol of oxygen. Therefore, the number of moles of NO that contains 1 mol of oxygen is also 1 mol.
Finally, we can use Avogadro's number to convert the number of moles of NO to the number of molecules of NO. Avogadro's number is approximately 6.02 x 10^23 molecules/mol. Therefore, the number of molecules of NO that contains 16 g of oxygen is:
number of molecules of NO = number of moles of NO x Avogadro's number
number of molecules of NO = 1 mol x 6.02 x 10^23 molecules/mol
number of molecules of NO = 6.02 x 10^23 molecules
Therefore, there are approximately 6.02 x 10^23 molecules of NO that contain 16 g of oxygen.
Can someone help! The diagram shows a cross section of the Earth near its surface.
In the diagram, continental crust is labeled ___, and oceanic crust is labeled ____
A. 1; 2
B. 3; 1
C. 2; 1
D. 1; 3

Answers
The thinner and denser layer labelled "2" in the diagram represents the marine crust, while the thicker and less dense layer labelled "1" represents the continental crust. Therefore, A. 1; 2 is the right response.
Which of the crust layers is thicker and less dense?The land on Earth is made up of the continental crust, which is less dense, thicker (between 35 and 70 km), and primarily composed of the rock granite. The majority of the ocean is made up of oceanic crust, which is denser, thinner (5–7 km), and primarily composed of the rock basalt.
The crust of the continent is dense or less dense.With a density of around 2.7 grammes per cubic cm, continental crust has a largely granitic composition and is slightly lighter than Oceanic crust has a density of roughly 2.9 to 3 grammes per cubic centimetre and is composed of basalt, which is richer in iron and magnesium than granite.
To know more about continental crust visit:-
https://brainly.com/question/30290659
#SPJ1
Help with chemistry??

Answers
Answer:
The first image = double replacement
Second = single replacement
Third = Decomposition
Fourth = Combination
Explanation:
1. a color from each pair is being swapped and replacing each other so there is a double replacement
2. only the blue and the red are changing places so it is single replacement
3. the pair is being separated (decomposed)
4. the red and green are coming together (combined)
determine each of the following of 0.065 M KOH solutin. milliliters of the KOH required to neutralize 40.0 mL of a 0.035 M H2sO4 solution express in 2 sig figs
Answers
The volume of the KOH required to neutralize 40.0 mL of a 0.035 M H2sO4 solution is 0.0215 L.
Concentration is the abundance of a constituent divided by way of the overall volume of an aggregate. several sorts of mathematical descriptions may be outstanding: mass concentration, molar concentration, variety concentration, and extent awareness.
Given
V1 =?
C₁ = 0.65 M
C₂ = 0.035 M
n -2
V2 = 40 ml = 0.04 L
C₁V₁ = C₂V₂
V₁ = C₂V₂/ C₁
= 0.035 * 0.04 / 0.065
= 0.0215 L
The concentration of a substance is the quantity of solute found in a given amount of solution. Concentrations are normally expressed in terms of molarity, defined because of the variety of moles of solute in 1 L of answer.
The Concentration of an answer is a measure of the quantity of solute that has been dissolved in a given amount of solvent or answer. A concentrated answer is one that has a rather huge quantity of dissolved solute.
Learn more about concentration here:-brainly.com/question/26255204
#SPJ1
g Ammonia has been studied as an alternative "clean" fuel for internal combustion engines, since its reaction with oxygen produces only nitrogen and water vapor, and in the liquid form it is easily transported. An industrial chemist studying this reaction fills a tank with of ammonia gas and of oxygen gas, and when the mixture has come to equilibrium measures the amount of water vapor to be . Calculate the concentration equilibrium constant for the combustion of ammonia at the final temperature of the mixture. Round your answer to significant digits.
Answers
\(\text{Ammonia has been studied as an alternative "clean" fuel for internal combustion}\)
\(\text{engines, since its reaction with oxygen produces only nitrogen and water vapor,}\)
\(\text{and in the liquid form it is easily transported. An industrial chemist studying this}\)
\(\text{reaction fills a} \ \mathbf{100 \ L }\ \text{tank with} \ \mathbf{8.6 \ mol} \ \text{of ammonia gas and} \ \mathbf{28 \ mol} \ \ \text{of oxygen gas, }\)
\(\text{to be} \ \mathbf{2.6\ mol} \ .\ \text{Calculate the concentration equilibrium constant for the combustion of}\)
\(\text{ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.}\)
Answer:
Explanation:
From the correct question above:
The reaction can be represented as:
\(\mathbf{4 NH_3_{(g)}+ 3O_{2(g)} \iff 2N_{2(g)}+ 6H_2O_{(g)} }\)
From the above reaction; the ICE table can be represented as:
\(\mathbf{4 NH_3_{(g)}+ 3O_{2(g)} \iff 2N_{2(g)}+ 6H_2O_{(g)} }\)
I (mol/L) 0.086 0.28 0 0
C -4x -3x +2x +6x
E 0.086 - 4x 0.28 - 3x +2x +6x
At equilibrium;
The water vapor = \(\dfrac{2.6 \ mol}{100 \ L} = 6x\)
\(x = \dfrac{2.6}{100} \times \dfrac{1}{6}\)
\(x = 0.00433\)
\(\text{equilibrium constant} ({k_c}) = \dfrac{ [N_2]^2 [H_2O]^6 }{ [[NH_3]^4] [O_2]^3 }\)
\(\implies \dfrac{(2x)^2 (6x)^6}{(0.086-4x)^4\times (0.28-3x)^3} \\ \\\)
Replacing the value of x, we have:
\(K_c = \dfrac{4 \times 46,656 \times x^8}{(0.086-4x)^4\times (0.28 -3x)^3} \\ \\ K_c = \dfrac{4 \times 46656 \times (0.00433)^8}{(0.06868)^4(0.26701)^3} \\ \\ K_c = \mathbf{5.4446 \times 10^{-8}}\)
\(K_c = \mathbf{5.5 \times 10^{-8} \ to \ 2 \ significant \ figures}\)
Part A
Measure and record the masses of all the metal strips you set out in front of the test tubes.
B IU x X2 10pt
iii
Imi
hili
ole
Test tube
Metal
Mass (g)
1
magnesium
2.
zinc
3
copper
magnesium
4
5
zinc
6
copper
Answers
This is a good answer:

How many mL of 14.5M lithium carbonate solution must be used to deliver 4.20 g of lithium ion
Answers
Answer:
10.3 g Li) / (6.9410 g Li/mol) x (1 mol Li3PO4 / 3 mol Li) / (0.750 mol/L Li3PO4) = 0.6595 L = 660. mL
Explanation:
Write the chemical symbol of the element with 35 protons?
Answers
Answer:
Bromine
Explanation:
Bromine is a chemical element with symbol Br and atomic number 35.