Which of the following is NOT a property of table sugar?
white
sweet
soft
dissolves in water
Answers
Related Questions
Which statement about the carbon cycle is most true?
A. Energy is destroyed when organic matter decays.
B. The total mass of carbon on Earth is always increasing.
C. Carbon is lost from the cycle when it enters the atmosphere.
D. Carbon changes form, but the total amount on Earth remains the same
Answers
Answer:
its a.
Explanation:
Convert a density of 55.3 lbs/ft3 into g/mL.
Answers
The density of 55.3 lbs/ft³ is 0.884 g/mL.
To convert density from pounds per cubic foot (lbs/ft³) to grams per milliliter (g/mL), we can use the following conversion factors:
1 pound = 453.59237 grams
1 foot = 30.48 centimeters (cm)
1 inch = 2.54 centimeters (cm)
1 milliliter (mL) = 1 cubic centimeter (cm³)
First, we convert pounds to grams:
55.3 lbs = 55.3 lbs * 453.59237 g/lb = 25050.364 grams
Next, we convert cubic feet to milliliters:
1 ft³ = (30.48 cm)³ = 28316.8466 cm³
1 cm³ = 1 mL
Finally, we calculate the density in g/mL:
Density = (25050.364 g) / (28316.8466 mL) ≈ 0.884 g/mL
Therefore, the density of 55.3 lbs/ft³ is approximately 0.884 g/mL.
Learn more about density from the given link
https://brainly.com/question/1354972
#SPJ11
List 5 physical properties of matter. How many atoms of each type of element in C6H12O6?
Answers
Atoms of each type- C 6, H 12, O 6, total of 24 atoms in one molecule
If you have a mass of 72 kg, and eat 333 g of pizza for dinner, what percent pizza are you. (record your answer to the nearest hundredth)
Answers
Note: in the International System of Units, The kilogram or kilogramme (symbol: kg) is the base unit of mass.
2nd Note:
1 gram = 0.001 Kilograms
72 kilograms converted to grams is 72,000 grams.
333 grams is 0.333 kilograms.
0.23976 is the pizza percentage.
Rounded: 0.24
Therefore, your answer is 0.24
. The United Arab Emirates is one of the most important tourist destinations. On one of its beaches, Laila and
Nouf are playing basketball outside in 300K weather and its volume is 100 ml. If they leave the ball outsideAnd the temperature drops down to 250 Celsius, what is the volume of the gas in the ball if the pressure remains constant.
Answers
The volume of the gas in the ball will decrease if the temperature drops down to 250 Celsius, assuming the pressure remains constant. The volume of the gas in the ball will be 83.3 ml.
According to Charles's law, the volume of a gas at constant pressure is directly proportional to its absolute temperature.
As the temperature decreases from 300K to 250K, the volume of the gas in the ball will decrease as well.
Assuming the pressure remains constant, the final volume of the gas can be calculated using the formula V2 = V1 x T2/T1, where V1 is the initial volume, T1 is the initial temperature in Kelvin, T2 is the final temperature in Kelvin, and V2 is the final volume of the gas.
Plugging in the values, we get V2 = 100 ml x 250K/300K = 83.3 ml.
Therefore, the volume of the gas in the ball will be 83.3 ml if the temperature drops down to 250 Celsius, assuming the pressure remains constant.
Learn more about Charles's law here:
https://brainly.com/question/16927784
#SPJ11
Which of the following is most likely to cause you to start a filtration over again?
A.
failure to use a stirring rod
B.
overflowing the top edge of the filter paper
C.
placing the tip of the funnel in the center of the beaker
D.
using too large a piece of filter paper
Answers
Answer:
overflow the top edge of the filter paper
The solubility of potassium sulfate in water is 16 grams per 100
milliliter at 50 degrees centigrade. The smallest amount of water
which will dissolve 4 grams of this substance at the same temperature
will be
Answers
The answer is 25 grams for this question
In three separate trials and under the same conditions, you measure a gas's density to be 2.865 kg/m^3, 2.852 kg/m^3, and 2.860 kg/m^3. the actual density of the gas is3.214 kg/m^3. evaluate the precision and accuracy of your measurement.
Answers
Out of given data, all data is close to actual value, so all are precise but only first data is very much close to actual value so it is accurate.
What is precision and accuracy?When measuring data, precision and accuracy are two crucial considerations. Both precision and accuracy indicate how closely a measurement comes to the real value.
But accuracy also indicates how closely a measurement resembles a known or recognized value, while precision indicates how repeatable a measurement is, even if it is far from the acceptable value. Out of given data, all data is close to actual value, so all are precise but only first data is very much close to actual value so it is accurate.
Therefore, out of given data, all data is close to actual value, so all are precise but only first data is very much close to actual value so it is accurate.
To learn more about precision and accuracy, here:
https://brainly.com/question/28289139
#SPJ1
What should you do if you do not observe any difference in the TLC after 20 minutes? What does this say about the reaction or the analytical method?
What is occurring chemically with the sodium bisulfite? What is the orange color and why does it go away with the sodium bisulfite treatment?
What if, upon cooling, no crystals form? What can you conclude about this observation? What should you do in this case?
Answers
If one does not observe any difference in the TLC after 20 minutes, it shows that the reaction was not carried out or did not take place. In such a case, one should repeat the reaction under optimal conditions.
In such a case, you should consider rechecking the reaction or the analytical technique used. This situation suggests that the reaction may be unsuccessful due to a technical issue such as failure to provide necessary conditions for the reaction to occur. It may also imply that the reaction being analyzed did not undergo any significant transformation, hence no difference was observed.
One can solve this problem by changing the solvent and considering the pH of the solution to provide optimal conditions for the reaction to occur. A more sensitive analytical method could also be employed to detect small changes or differences. The primary cause of the orange color is impurities present in the product, which are subsequently reduced to form the final product through sodium bisulfite treatment. When cooled, if no crystals form, it indicates that the product did not form, and the reaction did not take place.
This can result from an incorrect ratio of reactants, the purity of reagents, or incorrect reaction conditions. In such a case, one should repeat the reaction under optimal conditions.
To know more about optimal conditions visit:
https://brainly.com/question/30547120
#SPJ11
If the atomic number of an element is 6 and the atomic mass is 12.01, how many protons are there in the nucleus?
A. 12
B. 6
C. 24
D. 52
Answers
The atomic number of an element represents the number of protons in the nucleus. In this case, the atomic number is 6. Therefore, there are 6 protons in the nucleus of this element. The correct answer is B. 6.
The atomic number of an element represents the number of protons in the nucleus. In this case, the atomic number is 6, which means there are 6 protons in the nucleus. The atomic mass is the sum of the number of protons and neutrons in the nucleus. Since the atomic mass is 12.01 and the atomic number is 6, we can subtract 6 from 12.01 to get the number of neutrons. This gives us a neutron count of approximately 6.01.
Therefore, The answer is B. 6 protons are in the nucleus of this element.
Learn More about nucleus here :-
https://brainly.com/question/21083478
#SPJ11
Based on the Law of Conservation of Matter, why do you think the weight of Steel Wool would change the way it did?
Answers
Answer:
Because matter cannot be destroyed or created, It can change form through physical, chemical changes but the matter never gets destroyed or created.
Explanation:
suppose that 3.14 g of a silver salt ( agx ) is dissolved in 720.0 ml of water. a current of 2.81 a , applied for 805 s , is required to plate out all of the silver in solution. what is the mass percentage of silver in the salt?
Answers
The mass percentage of silver in the salt is 8.18%.
Given that 3.14 g of a silver salt (AgX) is dissolved in 720.0 mL of water. A current of 2.81 A, applied for 805 s, is required to plate out all of the silver in the solution.
We are required to determine the mass percentage of silver in the salt.Main answer:Let's first calculate the amount of silver deposited using Faraday's laws of electrolysis:
From Faraday's first law of electrolysis,mass of silver deposited = ItM / 96500where I = current = 2.81 A, t = time for which current is passed = 805 s, M = molar mass of silver = 107.87 g/mol and 96500 C = 1 mol of electrons.
So, mass of silver deposited = (2.81 A × 805 s × 107.87 g/mol) / 96500= 0.256 g.
Now, let's determine the mass percentage of silver in the salt.
Mass percentage of silver in the salt can be calculated as:mass percentage = (mass of silver / mass of AgX) × 100where mass of silver = 0.256 g and mass of AgX = 3.14 g.
Therefore, mass percentage = (0.256 g / 3.14 g) × 100= 8.18%
The given question is based on Faraday's laws of electrolysis, which helps us to determine the amount of metal deposited during electrolysis.
According to Faraday's laws of electrolysis, the amount of metal deposited is proportional to the quantity of electricity passed through the electrolyte.
Hence, we have used Faraday's laws to determine the amount of silver deposited by applying a current of 2.81 A for 805 s.
From the calculation, we have obtained that 0.256 g of silver has been deposited on the cathode. Now, we are required to determine the mass percentage of silver in the salt.
The mass percentage can be calculated as the ratio of the mass of silver deposited to the mass of AgX taken initially multiplied by 100.
From the calculation, we have obtained that the mass percentage of silver in the salt is 8.18%.Hence, the mass percentage of silver in the salt is 8.18%.
The mass percentage of silver in the salt is 8.18%. This answer has been calculated using Faraday's laws of electrolysis, which helps in determining the amount of metal deposited during electrolysis.
To know more about Faraday's laws of electrolysis visit:
brainly.com/question/1640558
#SPJ11
Hi does anyone know how to do question 4?
Please show working out thanks.

Answers
answer:
a)1.274 g b) 0.013 mole
Explanation:
a) Mass of 1 mol of CuSo4. 5H20=
\(63 + 32 + (16 \times 4) + (7 \times 1) + (16 \times 5)\)
=246g
no. moles of CuSo4. 5H2O=
\( \frac{mass}{mass \: of \: 1mole} \)
so moles
=
\( \frac{3.14}{246} \)
0.013 mole
in the equation
1 mole of sulphuric acid reacts to form 1 mole of CuSo4.5H2O
in other words
0.013 mole of sulphuric acid reacts to form 0.013 mole of CuSo4.5H2O
so mass of 1 mole (Mr) H2So4=
\((2 \times 1) + 32 + (16 \times 4)\)
98g
mass of sulphuric acid= mole × mass of 1 mole
so mass= 0.013 × 98
1.274 g
b) In the equation
1 mole of sulphuric acid reacts to form 1 mole of copper(ll) oxide
in other words
0.013 mole of sulphuric acid reacts to form 0.013 mole of copper (ll) oxide
so 0.013 mole of copper(ll) oxide is reacted
Hope it helped and pls mark brainliest
When a 0.3546 g of vanadium metal is heated in air, it reacts with oxygen to achieve a
final mass of 0.6330 g. Calculate the empirical formula of this vanadium oxide
Answers
Answer:
V2O5 is the empirical formula of vanadium oxide
Explanation:
The molecular mass of Vanadium is 50.949 g/mol
Number of moles of vanadium = 0.3456 g/50.949 g/mol = 0.00696 moles
The molecular mass of Oxygen is 16.0 g/mol
Number of moles of Oxygen = (0.6330 – 0.354) g/16 g/mol = 0.0174 moles
Emperical formula
V (0.00696 moles/0.00696 moles) O (0.0174 moles /0.00696 moles)
V 1 O2.5
Multiply by two get a whole number
V2 O5
Two substances are dissolved in water and start to react. Which statement accurately describes the appearance of the solution when it reaches equilibrium? (1 point)
A) No changes will be apparent, as the forward and reverse
reactions continue.
B) The appearance will continue to change as the forward and
reverse reactions continue.
C) The appearance will continue to change as the forward
reaction continues at a slow, balanced rate.
D) No changes will be apparent, as the reactions stop.
Answers
The statement that accurately describes the appearance of the solution when it reaches equilibrium is: no changes will be apparent, as the forward and reverse reactions continue.
WHAT IS EQUILIBRIUM?A chemical reaction is said to be at equilibrium when the rate of the forward reaction equals that of the reverse reaction.
In the case of a chemical reaction at equilibrium, formation of products equals the formation of reactants.
According to this question, if two substances are dissolved in water and start to react, no changes will be apparent, as the forward and reverse reactions continue.
Learn more about equilibrium reaction at: https://brainly.com/question/11114490
Answer:
1. The rate of the forward and backward reactions are equal.
2. No changes will be apparent, as the forward and reverse reactions continue.
3. The reactions that happen in baking are not reversible reactions.
4. The reaction mixture is not complete as reactants are still being added to the system.
5. in step 5 when the person returns to a healthy level of blood sugar and remains there
Explanation:
made a 100% on the assessment
A small positively charged ball is moved closer to a large, positively charged ball. which describes how the small ball likely responds when it is released?
Answers
Answer:
This is just my guess, but since opposites attract, then im guessing that alikes repel each other. So, they will go away from each other when the ball is released (I think).
Explanation:
Hope this helps! If it did, please mark it as brainliest! It would help a lot! Thanks! :D
Fill In The Blank, Non-ferrous metals typically become __________ when heated and quenched.
Group of answer choices
brittle
stronger
softer
harder
Answers
Non-ferrous metals typically become stronger when heated and quenched.
Non-ferrous metals are metals or alloys that do not include iron (or iron allotropes, such as ferrite, etc.) in significant quantities.
Non-ferrous metals are employed because they have desired qualities like reduced weight (for example, aluminium), greater conductivity (for example, copper), non-magnetic characteristics, or corrosion resistance (for example, zinc), even though they are often more expensive than ferrous metals. In the iron and steel sectors, several non-ferrous materials are also employed. Bauxite, for instance, is used as a flux in blast furnaces, whereas wolframite, pyrolusite, and chromite are utilised to create ferrous alloys.
To knwo more about Non-ferrous metals
https://brainly.com/question/33291477
#SPJ11
d
5 Aluminium and iron oxide (Fe₂O3) react together to produce aluminium oxide
(Al2O3). The equation for the reaction is:
Tran oxid
2:2
241+Fe2O3Al2O3+2Fe
Relative atomic masses (A): Al = 27,0=16, Fe=56.
Calculate the mass of iron that is produced by reacting 20 g of iron oxide with
an excess of aluminium.
Maths skills links
You may also need to convert the mass of a substance into an amount in moles
(and vice versa) when using moles to balance equations.
Answers
Aluminum and iron oxide (Fe₂O₃) react together to produce aluminum oxide, 20 g of Fe₂O3 will produce 14 g of Fe.
What is meant by oxide?Oxide is a category of chemical compound that has one or more oxygen atoms and also another element in its composition.
2Al + Fe₂O₃ -> Al₂O₃ + 2Fe
Molar mass of Fe₂O₃ is: (2 x 56) + (3 x 16) = 160 g/mol
moles of Fe2O3 = 20 g / 160 g/mol = 0.125 mol
As the reaction is carried out with an excess of Al, we can assume that all of the Fe₂O₃ is consumed and that 2 moles of Fe are produced for every mole of Fe₂O₃
moles of Fe = 2 x moles of Fe₂O₃ = 0.25 mol
Molar mass of Fe is : 56 g/mol
So the mass of Fe produced is:
mass of Fe = moles of Fe x molar mass of Fe
mass of Fe = 0.25 mol x 56 g/mol = 14 g
Therefore, 20 g of Fe₂O₃ will produce 14 g of Fe.
To know more about oxides, refer
https://brainly.com/question/30368235
#SPJ1
Walking at a brisk pace burns off about 280 cal/h. how long would you have to walk to burn off the calories obtained from eating a cheeseburger that contained 25 g of protein, 25 g of fat, and 31 g of carbohydrates? [hint: one gram of protein or one gram of carbohydrate typically releases about 4 calg, while fat releases 9 cal/g. ] hours
Answers
You would need to walk at a brisk pace for about 1 hour and 40 minutes to burn off the calories obtained from eating the cheeseburger.
25 g of protein and 31 g of carbohydrates release 4 cal/g, which equals 240 calories. 25 g of fat release 9 cal/g, which equals 225 calories. So, the total calories in the cheeseburger are 465.
Now, to burn off 465 calories at a rate of 280 cal/h, we need to divide 465 by 280, which equals 1.66 hours or approximately 1 hour and 40 minutes.
In summary, to burn off the calories obtained from a cheeseburger containing 25 g of protein, 25 g of fat, and 31 g of carbohydrates, you would need to walk at a brisk pace for about 1 hour and 40 minutes.
To know more about carbohydrates click on below link:
https://brainly.com/question/29775112#
#SPJ11
1. Starting with a 0. 1525 m hcl stock solution, three standard solutions are prepared by sequentially diluting 5. 00 ml of each solution to 100. 0 ml. What is the concentration of each solution?.
Answers
Each solution has a concentration of 7.625 x 103 m, 3.8125 x 104 m, and 1.90625 x 105 m, respectively.
Dilution is the process of lowering a sample's concentration by incorporating more solvent, according to its definition. The following is the dilution formula.
C₁V₁ = C₂V₂
where C1 is the sample's initial concentration.
V1 is the initial sample volume.
After dilution, the ultimate concentration is C2.
After dilution, the ultimate total volume is V2.
Calculate the concentration of the diluted solution by entering the numbers into the formula.
first common answer
C₁V₁ = C₂V₂
0. 1525 m(5.00 ml) = C₂(100.0 ml) (100.0 ml)
C₂ = 7.625 x 10⁻³ m
Second common solution
C₁V₁ = C₂V₂
7.625 x 10⁻³ m(5.00 ml) = C₂ (100.0 ml)
C₂ = 3.8125 x 10⁻⁴ m
Third accepted option
C₁V₁ = C₂V₂
3.8125 x 10⁻⁴ m(5.00 ml) = C₂(100.0 ml) (100.0 ml)
C₂ = 1.90625 x 10⁻⁵ m
To learn more about dilution Visit:
brainly.com/question/1615979
#SPJ4
6. For a chemical reaction, the heat of reaction is equal
to the
Answers
Answer:
internal energyExplanation:
At constant pressure, the heat of reaction is equal to the enthalpy change of the system. Most chemical reactions occur at constant pressure, so enthalpy is more often used to measure heats of reaction than internal energy
For a chemical reaction, the heat of reaction, also known as enthalpy change (∆H), is equal to the difference between the total energy of the products and the total energy of the reactants.
Enthalpy change represents the amount of heat energy exchanged or released during the course of a reaction. The heat of reaction can be positive (endothermic), indicating that the reaction absorbs heat from the surroundings, or negative (exothermic), indicating that the reaction releases heat to the surroundings.
The magnitude of the heat of reaction is a measure of the amount of heat energy involved in the reaction.
Learn more about Heat of reaction, here:
https://brainly.com/question/30464598
#SPJ2
If element X has 5 valence electrons, what would you expect it to do to fulfil its octet? pls help

Answers
Answer:
a
Explanation:
Answer:
Gain 3 electrons.
Explanation:
If element X have 5 valence electrons it will more than likely gain 3 electrons to fill its octet to become stable. Gaining the 3 electrons is much easier for the atom than if it were to give away it's 5 valence electrons. Elements with 5 valence electrons that could represent element X are:
NitrogenPhosphorusArsenicAntimonyWhat is the correct formula for Triphosphorous hexachloride?
Answers
Answer:
P3
Explanation:
Im pretty sure hope this helps
When naming amines according to the iupac system, the e in the corresponding alkane is replaced with?
Answers
The iupac system, the e in the corresponding alkane is replaced with -amine
What is IUPAC system?The International Union of Pure and Applied Chemistry's (IUPAC) suggested nomenclature for organic chemistry is a way to name chemical substances that are classified as organics. The article appears in the Nomenclature of Organic Chemistry.
Why is the IUPAC system important?IUPAC nomenclature is significant because it establishes a uniform system for naming chemical substances.
The IUPAC nomenclature system was developed with the goal of creating a global consensus on compound names to improve communication. The system's aim is to associate each name with a distinct and clear structure, giving each structure a name that is both unique and unambiguous.
To know more about IUPAC system visit:
https://brainly.com/question/23036094
#SPJ4
How does the conductivity of metalloids compare to the conductivity of metals and nonmetals?
Metalloids conduct electricity better than metals, but not as well as nonmetals.
Metals, metalloids, and nonmetals all have the same level of conductivity.
Metalloids conduct electricity better than nonmetals, but not as well as metals.
Metalloids cannot conduct electricity.
Answers
The conductivity of metalloids can be compared to the conductivity of metals and nonmetals because metalloids conduct electricity better than nonmetals, but not as well as metals (Option C).
What are metalloids?The expression metalloids is a term used to denote chemical elements that have features resembling metals such as an acceptable electrical conductivity, but they are not metals (e.g. boron, germanium, antimony, arsenic, polonium, etc).
Therefore, with this data, we can see that metalloids are similar to metals in electrical conductivity but they are not metals because they do not fit all properties of metals.
Learn more about metalloids here:
https://brainly.com/question/6007181
#SPJ1
How cold does it really get on Pluto's moon, Charon? (I know this is not a Chem question per se, but is there no Earth Science, nor Space, nor Astronomy, nor General Science category on Brainly?)
Answers
Answer:
Explanation:
After various studies and experiments conducted on Pluto's moon, Charon it has been determined that it gets to minus 459.67 degrees Fahrenheit which would be equivalent to minus 273.15 degrees Celsius. This is the coldest temperature that scientists have registered for Charon. This temperature is nearing absolute zero and puts it colder than Pluto itself since Pluto's temperature is believed to get to minus 400 degrees Fahrenheit which is equivalent to minus 240 degrees Celcius.
what is the difference between liquid nitrogen and nitrogen gas
Answers
Answer:
The difference between liquid nitrogen and nitrogen gas is liquid nitrogen gas is man made or caused by humans but, nitrogen gas takes place naturally in the earths atmosphere.
Explanation:
Hope this helps :)
The difference between liquid nitrogen and nitrogen gas is their physical state at different temperatures and pressures. Nitrogen gas is the gaseous form of nitrogen at room temperature and standard pressure, while liquid nitrogen is the extremely cold liquid form of nitrogen achieved at very low temperatures.
Liquid nitrogen and nitrogen gas are both forms of the chemical element nitrogen, but they exist in different physical states due to differences in temperature and pressure.
Nitrogen Gas;
Nitrogen gas is the gaseous form of nitrogen at room temperature and standard atmospheric pressure.
It is a colorless, odorless, and non-flammable gas.
Nitrogen gas makes up about 78% of Earth's atmosphere and is an essential component for life.
It is often used in various industrial processes, such as purging, inerting, and blanketing, due to its non-reactive nature.
Liquid Nitrogen;
Liquid nitrogen is the liquid state of nitrogen achieved at extremely low temperatures.
It is obtained by cooling nitrogen gas to its boiling point, which is approximately -196 degrees Celsius (-321 degrees Fahrenheit) at atmospheric pressure.
Liquid nitrogen is extremely cold and can cause severe frostbite or freezing of tissues upon contact with skin.
It is used in a wide range of applications, including cryopreservation of biological samples, cooling of sensitive equipment, and in the culinary industry for instant freezing.
To know more about liquid nitrogen here
https://brainly.com/question/4492682
#SPJ2
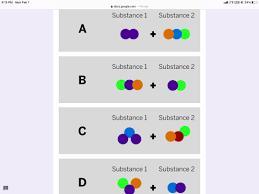
Starting substances
Substance 1
?
Substance 2
+ ?
Jamie works at a company that makes cleaning chemicals. She is trying to make a chemical that smells like flowers. She took two
samples that were gases at room temperature and mixed them in a sealed container.
The diagram above shows the repeating groups of atoms that make up the two starting substances.
After mixing, Jamie found two substances that smelled like flowers in the sealed container. (Nothing had escaped.)
Which of the diagrams shows the repeating groups of atoms that make up the ending substances?
Answers
The combination of the compounds can be found in option C
How is a compound formed?
A compound is formed through a chemical reaction or a combination of elements. In a chemical reaction, two or more elements combine or react with each other to form a compound.
The elements involved in the reaction undergo a rearrangement of their atoms and bonding to form new chemical bonds, resulting in the formation of a compound with different properties from the original elements. This is clear from the images that have been shown in the question.
Learn more about compound:https://brainly.com/question/14117795
#SPJ1

In the following equation, ______ is being oxidized and ______ is being reduced.
CO3 2- + 2H+ → CO2 + H2O
A. None of these
B. carbon, oxygen
C. carbon, hydrogen
D. hydrogen, carbon
Answers
\(oxidation \: number \: of \: oxygen = \\ before \: rxn = - 2 \\ after \: rxn = - 2\)
\(oxidation \: number \: of \: hydrogen = \\ before \: rxn = + 1 \\ after \: rxn = \\ 2x - 2 = 0 \\ x = + 1\)
\(oxidation \: number \: of \: carbon = \\ before \: rxn = \\ x - 6 = - 2 \\ x = 4 \\ after \: rxn = \\ x - 4 = 0 \\ x = 4\)
Option A\(oxidation \: numbers \: remain \: constant \\ so \: none \:a re \: undergoing \: oxidation \: \\ nor \: reduction \: \)
Answer:
D
Explanation:
I believe that is the answer
Question 2
Use the balanced chemical reaction below to answer the question.
200 + O2 + 2CO2
What is the ratio of moles of oxygen used to moles of carbon dioxide
produced?
O 2 mol O2: 2 mol CO2
O 1 mol O2: 1 mol CO2
O 2 mol O2: 1 mol CO2
O 1 mol O2:2 mol CO2
