which of the following is a true statement? which of the following is a true statement? evaporation of water is an endothermic process where δh is negative. a combustion reaction is an exothermic process where δh is negative. freezing of water is an exothermic process where δh is positive. melting of ice is an endothermic process where δh is negative.
Answers
The correct statement is combustion reaction is an exothermic process where δh is negative.
Chemical reactions with a negative H are known as exothermic reactions because they generate heat. In other words, the energy that the reaction releases is greater than the activation energy required to start the reaction.Neutralization, the Haber process, the thermite reaction, and combustion reactions are a few examples of exothermic reactions.An endothermic reaction is the opposite of an exothermic reaction. Endothermic reactions generate less heat than they take in from their surroundings. Exergonic and endergonic reactions are subtypes of exothermic and endothermic reactions, respectively. In exergonic and endergonic reactions, the net energy—whether it be heat, light, or sound—is greater (exergonic) or lower (endergonic) than the energy required for the reaction to proceed.To understand more about endothermic reaction -
https://brainly.com/question/23184814
#SPJ4
Related Questions
the general formula for an amino acid is: rchcooh rnh2coh rchnh2cooh rnh2ooh
Answers
The general formula for an amino acid is RCH(NH2)COOH. The R group can vary among different amino acids, giving each amino acid its distinct properties.
Amino acids are organic compounds that serve as the building blocks of proteins. The general formula for an amino acid consists of four components: R, CH(NH2), COOH.
R represents the side chain or the variable group, which differs among different amino acids and gives each amino acid its unique properties. It can be a simple hydrogen atom (H) or a complex organic group.
CH(NH2) represents the amino group (-NH2) attached to the carbon (C) atom. It consists of one carbon atom bonded to three hydrogen atoms (CH3) and an amino group (NH2).
COOH represents the carboxyl group (-COOH) attached to the carbon atom. It consists of a carbon atom double-bonded to an oxygen atom (C=O) and a hydroxyl group (-OH).
The combination of the amino group and the carboxyl group on the same carbon atom forms an amino acid.
The general formula for an amino acid is RCH(NH2)COOH. This formula represents the structure of an amino acid, with R representing the side chain, CH(NH2) representing the amino group, and COOH representing the carboxyl group. The R group can vary among different amino acids, giving each amino acid its distinct properties.
To know more about amino acid visit:
https://brainly.com/question/14351754
#SPJ11
Freddie is carrying out an experiment for which he has to maintain a water bath at a temperature of 37°C. He has a choice of four thermometers, all of which read from 0 to 100°C.Thermometer A is marked in 5°C intervals, B in 1°C intervals, C in 10°C intervals and D in 20°C intervals. Which thermometer should Freddie choose to monitor the water bath's temperature?
B
D
A
C
Answers
Answer:
C
Explanation:
because 37 does not end with 5 or 0, so it'd be harder to see if the water temperature is accurate if your thermometer goes up by 5s or 10s. And using D, the one that goes up by 20s is out of the question, it'd be too difficult to read. Using a thermometer that goes up by 1s is just best because you can be positive when the water is at 37°C
which molecule has a central atom that is sp3 hybridized? select the correct answer below: sf6 ch3cl alcl3 pcl5
Answers
The molecule that has a central atom that is sp3 hybridized is CH3Cl. To determine the hybridization of an atom, we need to count the number of electron groups around the central atom.
In this case, the central atom in CH3Cl is carbon (C). CH3Cl has four electron groups around the central carbon atom: three sigma bonds with hydrogen (C-H bonds) and one sigma bond with chlorine (C-Cl bond). Each sigma bond counts as one electron group.
The four electron groups indicate that the carbon atom is sp3 hybridized. In sp3 hybridization, the carbon atom forms four sigma bonds with four electron groups, resulting in a tetrahedral geometry. Therefore, the correct answer is CH3Cl.
More on sp3 hybridized: https://brainly.com/question/8235897
#SPJ11
If we increase the temperature of the vessel to 450 K at constant volume, what would the pressure inside the vessel be?
Group of answer choices
10 atm
5 atm
20 atm
15 atm
Answers
If we increase the temperature of the vessel to 450 K at constant volume, then the pressure inside the vessel be 15 atm.
The relationship between temperature and pressure for a fixed volume is described by Gay-law. According to this Law, the pressure of a gas is precisely proportional to its temperature for a fixed volume of the gas. It is written mathematically as P ∝ T, where P is the gas pressure.
T is the gas's temperature (in Kelvin).
P = kT, where k is the proportionality constant, and P / T is equal to k.
In this scenario, the vessel's temperature rises from 300 K to 450 K, its volume doesn't change, and its internal pressure rises.
If the temperature doubles, the pressure will also double.
To learn more about Gay-Lussac law, refer:
brainly.com/question/2644981
#SPJ4
Cifras significativas de 63,000
Answers
Answer:
Cifras significativas de 63,000
Result 63000
Sig Higos 2 (63000)
Decimales 0
Notación cientifica 6.3 × 104
Notación electrónica 6.3e+4
Palabras sesenta y tres mil
How does doubling [Base] affect the rate of an E1 reaction? Select the single best answer. rate quadrupled rate doubled no change rate halved
Answers
There is "no change" in the rate of an E1 reaction when you double the base.
Doubling the base concentration in an E1 reaction will not have a significant impact on the reaction rate. In an E1 reaction, the rate-determining step is the formation of a carbocation intermediate via the departure of a leaving group. This process is unimolecular and depends solely on the concentration of the substrate.
The role of the base in an E1 reaction is to act as a nucleophile in the subsequent step, which involves deprotonating the carbocation intermediate to form the product. Since the rate-determining step is not directly affected by the concentration of the base, doubling the base concentration will not change the overall reaction rate. Therefore, the best answer is "no change."
Learn more about E1 reaction here: https://brainly.com/question/30887510
#SPJ11
Glacier striations (scrapings) were found in the deserts of Africa. What did Wegener suggest about these deserts long ago?
Answers
Wegener suggested that the deserts of Africa were once covered in ice and that the striations were formed by the movement of the ice across the desert floor.
Glacier striations are grooves or scratches in the bedrock that have been created by a glacier sliding over the surface. They are typically found in areas where the ice has melted away, revealing the bedrock beneath.
Wegener suggested that the deserts were once wetter and lusher. He proposed that the climate has gradually changed over time, making the deserts arider. This is supported by evidence of ancient riverbeds and lakes in the Sahara Desert.
Glacier striations can be used to help researchers learn about the history of a particular glacier and the climate in the area where it is located. For example, the direction of the striations can indicate the direction the glacier was moving and the depth of the striations can give clues about how deep the glacier was.
To know more about glaciers, click below:
https://brainly.com/question/6666513
#SPJ9
Which element has higher electronegativity: Nitrogen or Arsenic
Answers
Answer:
Definetly Nitrogen
Explanation:
Nitrogen is the most electronegative
compare solution a and solution b in each label and drag the label to the appropriate concentration description.
olution A = 0.5mM and Solution B = 100mm
Solution A = 2 mM of n+3 and Solution B = 1 M n+1
Solution A = 3.5mg/dL and Solution B = 3.7mg/dL
Solution A = 0% (w/v) and Solution B = 0% (w/v)
Solution A = 10-3 M and Solution B = 10-5M
Solution A = 12.23% (w/v) and Solution B = 12.3% (w/v)
Substance A is more conentrated than B
Equal concentrations
Substance B is more concentrated than A
Answers
A) Solution B of 100 mM is more concentrated than Solution A of 0.5 mM.
B) Solution B of 3.7mg/dL is more concentrated than Solution A of 3.5mg/dL.
C) Solution A = 0% (w/v) and Solution B = 0% (w/v) equal concentrations.
D) Solution A of 10⁻³ M is more concentrated than Solution A of 10⁻⁵M.
E) Solution B of 12.3% (w/v) is more concentrated than Solution A of 12.23% (w/v)
What is concentration?In chemistry, concentration can be described as the abundance of components divided by the total volume of a solution. Several kinds of mathematical descriptions can be distinguished: mass concentration, number concentration, molar concentration, and volume concentration.
The concentration is defined as the kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar concentration has variants, such as osmotic concentration and normal concentration.
Molarity, molality, Normality, and weight/ volume percentage are also used to define the concentration of the solution.
Learn more about concentration, here:
https://brainly.com/question/10935668
#SPJ1
A molecule of glucose is comprised of 6 atoms of carbon, 12 atoms of hydrogen, and 6
atoms of oxygen. What is the molar mass of glucose?
Answers
Answer:
48
Explanation:
because you add 6 and 6 and 12 to get it
sterile isopropyl alcohol (ipa) bottles did not arrive in the supply order. what action should compounding personnel take?
Answers
Compounding personnel should contact the supplier to follow up on the delivery of the sterile isopropyl alcohol (IPA) bottles that did not arrive in the supply order. The personnel should request an estimated time of arrival and make sure to provide the exact details of the order.
If the sterile isopropyl alcohol (IPA) bottles did not arrive in the supply order, the compounding personnel should take the following steps:
It is important for the compounding personnel to take swift action in this situation to ensure that the necessary supplies are obtained and that compounding activities can continue without disruption.
know more about isopropyl alcohol here
https://brainly.com/question/14896958#
#SPJ11
Which statement about exothermic reactions is TRUE?
A.In an exothermic reaction, energy is absorbed, bonds are broken, and the temperature decreases.
B. In an exothermic reaction, energy is released, bonds are formed, and the temperature increases.
Answers
Which statement about exothermic reactions is TRUE?
A.In an exothermic reaction, energy is absorbed, bonds are broken, and the temperature decreases.
B. In an exothermic reaction, energy is released, bonds are formed, and the temperature increases.How many grams does 5.60 x 10 to the 22 molecules of SiO2 weigh
Answers
Answer:
Multiply n by M to convert to the mass, m:
m = n × M = (0.09299 mol)/1 × (60.083 g)/(1 mol) = 5.5871 g
Explanation:
Convert from molecules into grams:
5.6×10^22 molecules silicon dioxide (SiO2)
Determine the number of molecules, N_molecules:
N_molecules = 5.6×10^22 molecules
Look up Avogadro's constant, N_A, to find the number of molecules in a mole:
N_A = 6.022×10^23 molecules/mol
Divide N_molecules by N_A to convert to the amount of substance, n:
n = N_molecules/N_A = N_molecules/1 × 1/N_A = (5.6×10^22 molecules)/1 × (1 mol)/(6.022×10^23 molecules) = 0.09299 mol
Calculate the molar mass, M, of SiO_2:
M = 60.083 g/mol
Conduct research to examine the following factors regarding the storage of nuclear waste.
the costs, risks, and benefits to building a nuclear waste storage facility beneath Yucca Mountain
the costs, risks, and benefits to building a nuclear waste storage facility somewhere else
the costs, risks, and benefits of not building a nuclear waste storage facility at all
Based on the data you have compiled, propose an appropriate solution to this problem. Use your data to support your position on the issue.
Answers
In order to reduce the risk of radiation exposure to individuals and environmental contamination, radioactive wastes are kept. The wastes' radioactivity decreases over time.
What are the biggest problems with keeping radioactive waste in storage for a long time?Large steel and concrete barrels that contain the garbage are typically properly sealed, although accidents and leaks can still happen. Cancerous growths can result from the severe negative impacts of nuclear waste on life.
How is radioactive waste stored?Currently, dry casks are used to store all of the nuclear waste that a power plant produces over the course of its lifetime. Since 1987, Yucca Mountain in Nevada has been intended as a permanent disposal location for spent nuclear material.
To know more about radioactive wastes visit :-
https://brainly.com/question/9816140
#SPJ1
Which of the following is the most affected in people with sickle-cell anemia? O the partial pressure of oxygen in air
O the vol % of CO2 in blood
O the partial pressure of CO2 in the tissues
O the partial pressure of CO2 in the lungs O the acidity of the blood plasma
O the acidity inside the red blood cells O the Bunsen solubility coefficient for oxygen O chloride shift
Answers
The most affected factor in people with sickle-cell anemia is the partial pressure of oxygen in the tissues.
Sickle-cell anemia is a genetic disorder that affects the structure of red blood cells. It causes the production of abnormal hemoglobin, known as hemoglobin S, which can distort the shape of red blood cells and make them rigid and prone to sticking together. This can result in reduced oxygen delivery to tissues and organs.
The most affected factor in people with sickle-cell anemia is the partial pressure of oxygen in the tissues. Due to the abnormal shape and reduced flexibility of sickle cells, they can get stuck in small blood vessels, leading to poor oxygen supply to tissues. This can cause tissue damage, pain, and other complications associated with sickle-cell anemia.
Other factors listed, such as the partial pressure of oxygen in air, the vol % of CO2 in blood, the partial pressure of CO2 in the lungs, the acidity of the blood plasma, the acidity inside the red blood cells, the Bunsen solubility coefficient for oxygen, and the chloride shift, may be influenced to some extent by sickle-cell anemia but are not the primary factors most affected by the condition.
In people with sickle-cell anemia, the partial pressure of oxygen in the tissues is the most affected factor. The abnormal red blood cells in sickle-cell anemia can cause reduced oxygen delivery to tissues, leading to various complications associated with the condition.
To know more about oxygen visit ,
https://brainly.com/question/30284244
#SPJ11
What is evaporation ?In what way it is different from boiling? What is the effect
of humidity on the rate of evaporation?
Define Latent heat of fusion? Why does steam cause more severe burning as
compared to boiling water at 1000C ?
Answers
Evaporation is the process by which a liquid converts into a gas or vapor at a temperature below its boiling point. Latent heat of fusion is the amount of heat required to convert a solid into a liquid without any change in temperature. Steam causes more severe burning as compared to boiling water at 100°C due to the large amount of latent heat of vaporization.
On the other hand, boiling is the process by which a liquid turns into a gas or vapor at a temperature equal to or above its boiling point. In other words, when the liquid is heated to a temperature equal to or greater than its boiling point, it turns into a gas or vapor. The effect of humidity on the rate of evaporation is that if the air around the liquid is already humid, then the rate of evaporation will be slower because the air is already saturated with water molecules.
Latent heat of fusion is the amount of heat required to convert a solid into a liquid without any change in temperature. In other words, it is the amount of heat required to break the intermolecular forces between the molecules of a solid to convert it into a liquid.
Steam causes more severe burning as compared to boiling water at 100°C because steam contains a large amount of latent heat of vaporization. When steam comes in contact with the skin, it releases a large amount of latent heat of vaporization, which causes more severe burns as compared to boiling water at 100°C.
Evaporation is the process of turning a liquid into a gas or vapor at a temperature below its boiling point. Boiling, on the other hand, is the process of turning a liquid into a gas or vapor at a temperature equal to or above its boiling point. Humidity affects the rate of evaporation. Latent heat of fusion is the amount of heat required to convert a solid into a liquid. Steam causes more severe burning as compared to boiling water at 100°C due to the large amount of latent heat of vaporization.
To know more about Evaporation visit:
brainly.com/question/28319650
#SPJ11
City A in the Southern Hemisphere and City B in the Northern Hemisphere are located at the same latitude. Which statement is likely true about these
cities?
City B has the larger annual temperature range.
Both cities should have nearly identical winter temperatures.
City A has the larger annual temperature range.
Both cities likely have the same annual temperature range.
Answers
Answer:
City B has the larger annual temperature range
Explanation:
This is correct option because generally the northern side of the equator is high in temperature than the southern hemisphere part.
Since the southern side of the equator or Southern Hemisphere, where city A resides will generally have higher altitude or rise, so this creates higher average temperature.
A girl has a weight of 450 N and her feet have a total area of 300Cm2
Answers
Answer:
15000N/m²
Explanation:
Given parameters:
Force exerted by the girl = 450N
Area = 300cm²
Unknown:
Pressure exerted by her feet =?
Solution:
Pressure is the force per unit area on a body;
Pressure = \(\frac{force }{area}\)
We need to convert the given area to m²;
10000cm² = 1m²
300cm² = \(\frac{300}{10000}\) = 0.03m²
Pressure = \(\frac{450}{0.03}\) = 15000N/m²
how can i find wavelength in a wave?
Answers
Wavelength (L) is calculated using: L = gT²/2π, here g=9.8 m/s2 and T is wave period in seconds.
What is wavelength?Wavelength of a wave describes how long the wave is and the distance from the "crest" (top) of one wave to the crest of next wave is called wavelength. We can also measure from the "trough" (bottom) of one wave to trough of next wave and get the same value for the wavelength.
We measure wavelength in following ways:
Use photometer to measure the energy of wave.
Convert energy into joules (J).
Divide energy by Planck's constant, 6.626 x 10⁻³⁴, to get the frequency of wave.
Divide speed of light, ~300,000,000 m/s, by frequency to get wavelength.
To know more about wavelength, refer
https://brainly.com/question/10750459
#SPJ9
What is the maximum number of moles of so3 that can be produced by the reaction of 2. 0 mol of s with of o2?
Answers
The maximum number of moles of SO₃ that can be produced by the reaction of 2.0 mol of S with of O₂ will be 2, two moles.
What are moles?Moles are the quantity of a material in per unit of solution. It is the SI unit of amount of a substance. The moles can be calculated by dividing the molecular weight of an object with the molecular mass of the substance.
The balanced reaction is
2SO₂ +O₂ →2SO₃
The ratio is 2:1
So if 2.0 mol of sulfur will reaction with 1 mole of oxygen there will be 2 moles of SO₃. The reaction clears the moles of SO₃ in the reaction.
Thus, maximum number of moles of SO₃ will be 2.
To learn more about moles, refer to the link:
https://brainly.com/question/15209553
#SPJ4
A gas mixture contains each of the following gases at the indicated partial pressure. N2 219 torr O2 106 torr He 244 torr What is the total pressure of the mixture? Express your answer in torr to three significant figures.
Answers
In this case, the partial pressures of nitrogen (N2), oxygen (O2), and helium (He) are given as 219 torr, 106 torr, and 244 torr, respectively. The total pressure of the gas mixture is 569 torr.
The total pressure of a gas mixture is the sum of the partial pressures of its individual components. In this case, the partial pressures of nitrogen (N2), oxygen (O2), and helium (He) are given as 219 torr, 106 torr, and 244 torr, respectively.
To find the total pressure, we simply add these partial pressures together:
Total pressure = Partial pressure of N2 + Partial pressure of O2 + Partial pressure of He
= 219 torr + 106 torr + 244 torr
= 569 torr
Therefore, the total pressure of the gas mixture is 569 torr.
To learn more about pressure, click here:
brainly.com/question/24719118
#SPJ11
How do you calculate % transmission
Answers
Answer:
you will have to use T = 1/10
What letter represents the trough? :) PLEASE FAST!
1) A
2) B
3) C
4) D
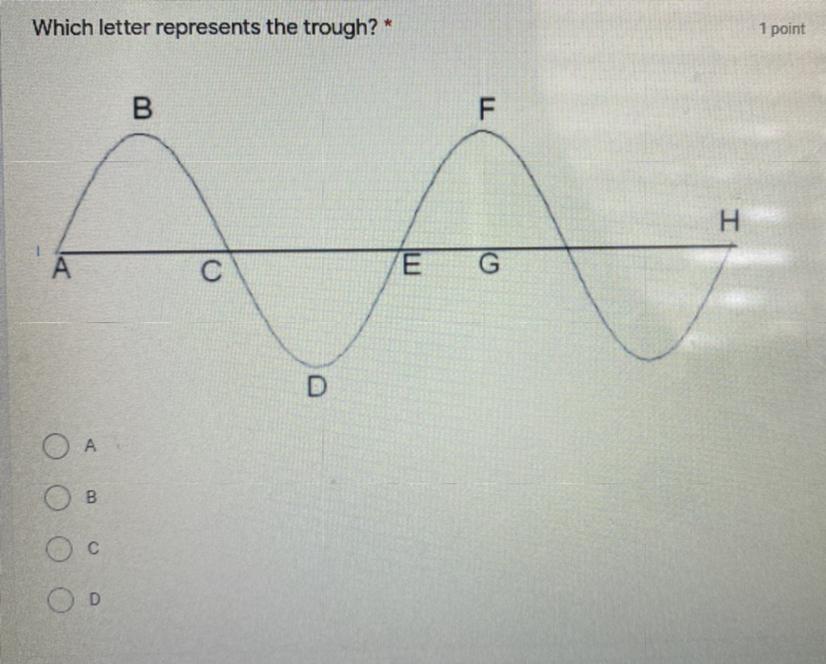
Answers
100 POINTS HELP WILL GIVE BRAINLIEST

Answers
Answer: 20 C
Explanation:
I would put it in 20 C because it is closer to the 20 C than the 30 C (it is below 25 C).
i need help with chemistry asap. the copounds Ch3Oh and Ch3CooH react in the presence of an acid catalyst to form. A. a carboxylic B. an aldehyde C. a ketone and D. an ester.
Answers
a flexible container at an initial volume of 8.15 l contains 9.51 mol of gas. more gas is then added to the container until it reaches a final volume of 15.5 l. assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container.
Answers
The number of moles added to increase the volume of the gas from 8.51 L to 15.5 L is 8.67 moles.
The initial volume of the gas is 8.15 L and final volume of gas is 15.5 L and the initial moles are 9.51 moles.
As per the Ideal gas equation,
PV = nRT
where,
P is pressure,
V is volume of gas,
n is the number of moles of gas,
R is the gas constant,
T is the temperature of the gas.
Rearranging the equation,
V/n = RT/P
The left side is constant, so, it is valid to see,
V₁/n₁ = V₂/n₂
V₁ is initial volume,
n₁ is the initial moles,
V₂ is the final volume,
n₂ is the final moles.
So, we can write this by putting the values,
8.51/(9.5) = 15.5/n₂
n₂ = 18.17
The number of moles added = n₁ - n₂
Added moles = 8.67 moles
The moles added are 8.67 moles.
To know more about Ideal gas equation, visit,
https://brainly.com/question/25290815
#SPJ4
What are the 4 methods that can be used to separate a mixture? Briefly explain and give an example of each.
Answers
Answer:
filtration : eg mixture of chalk and water
sieving: eg mixture of solids of different sizes
distillation: eg mixture of garri and water
crystallization: eg mixture of salt and water
Consider a nonadiabatic well-stirred reactor with simplifi ed chemistry, i.e., fuel, oxidizer, and a single product species. the reactants, consisting of fuel (yf = 0.2) and oxidizer (yox = 0.8) at 298 k, fl ow into the 0.003-m3 reactor at 0.5 kg / s. the reactor operates at 1 atm and has a heat loss of 2000 w. assume the following simplifi ed thermodynamic properties: cp = 1100 j / kg-k (all species), mw = 29 kg / kmol (all species), hf f o , = −2000 kj/ kg, hf ox o , = 0, and hf o , pr = −4000 kj/ kg. the fuel and oxidizer mass fractions in the outlet stream are 0.001 and 0.003, respectively. determine the temperature in the reactor and the residence ti
Answers
The first step is to calculate the molar flow rate of fuel, oxidizer, and product. This is done by dividing the mass flow rate (0.5 kg/s) by the molecular weight of each species (29 kg/kmol).
What is molecular?Molecular is a term used to describe the smallest units of matter. Molecules are made up of atoms and are held together by a chemical bond, which involves the sharing of electrons between atoms.
This gives us the following molar flow rates:
Fuel: 0.017 kmol/s
Oxidizer: 0.027 kmol/s
Product: 0.046 kmol/s
Next, we need to calculate the enthalpy change for the reaction. Since we are dealing with a single product species, the enthalpy change can be calculated using the following equation:
ΔH = (hf f o , + hf ox o , - hf o , pr) * n
Where:
hf f o , = Enthalpy of formation of fuel
hf ox o , = Enthalpy of formation of oxidizer
hf o , pr = Enthalpy of formation of product
n = Molar flow rate of product
Substituting the given values, we get the following:
ΔH = (-2000 + 0 - (-4000)) * 0.046 = 920 kJ/s
Now we can calculate the heat of reaction by multiplying the enthalpy change with the molar flow rate of the reactants. This gives us the following result:
Heat of reaction = (0.017 kmol/s * 920 kJ/s) + (0.027 kmol/s * 920 kJ/s) = 24.12 kJ/s
We can then calculate the temperature of the reactor by subtracting the heat loss (2000 W) from the heat of reaction and dividing by the total mass flow rate of the reactants (0.5 kg/s) multiplied by the specific heat capacity (1100 J/kg-K). This gives us the following result:
T = (24.12 kJ/s - 2000 W) / (0.5 kg/s * 1100 J/kg-K) = 436 K
Finally, we can calculate the residence time by dividing the volume of the reactor (0.003 m3) by the total mass flow rate of the reactants (0.5 kg/s). This gives us the following result:
Residence time = 0.003 m3 / 0.5 kg/s = 0.006 s
To learn more about molecular
https://brainly.com/question/26388921
#SPJ4
The temperature in the reactor is approximately 10.74 K. and 0.006 s.
The temperature in the reactor and the residence time, we need to solve the following set of equations:
dU = w + Q / m
Next, we need to find the rate of change of mass flow rate, which is given by:
dm = Fv - D
here Fv is the volume flow rate of reactants and D is the diffusion rate of the product.
Finally, we can use the above equations to find the temperature in the reactor and the residence time as follows:
Temperature in the reactor:
T = (dU / Q) / (m / cP)
here cP is the specific heat at constant pressure.
Residence time:
t = (m / D)
We can assume that the reactants have a volume flow rate of 0.5 kg/s and the product species has a volume flow rate of 0.001 kg/s. Therefore, the mass flow rate of the reactants is:
m = 0.5 kg/s * 0.002 m3/kg = 0.001 kg/s
The diffusion rate of the product can be calculated as:
D = k * (yox - yf) / (yf + yox)
here k is the reaction rate constant and (yox - yf) / (yf + yox) is the molar fraction of the product species.
Using the values of k, m, and (yox - yf) / (yf + yox) from the problem statement, we can calculate the diffusion rate of the product as:
D = 1 * (0.003 - 0.2) / (0.2 + 0.003)
= 0.00006 / 0.003
= 0.1833
Therefore, the residence time of the reactor is:
t = (0.001 kg/s / 0.1833 kg/mol) = 0.051 s
The temperature in the reactor is given by:
T = (dU / Q) / (m / cP)
here cP is the specific heat at constant pressure of the reactants, which is 1100 J/kg-K.
T = (w + Q / m) / (0.001 kg/s / 1100 J/kg-K) / (0.001 kg/s / 0.003 m3/kg)
= 10.74 K
Residence time = 0.003 m3 / 0.5 kg/s = 0.006 s
Therefore, the temperature in the reactor is approximately 10.74 K and 0.006 s.
Learn more about reactor visit: brainly.com/question/27823859
#SPJ4
what is the percentage by mass of hydrogen (H) in lithium hydroxide (LiOH)
Answers
41.7% is the percentage by mass of hydrogen (H) in lithium hydroxide (LiOH). The solution mixture is frequently defined in terms of mass percentage.
What is percentage by mass?The mass percent might be used to denote a degree. Furthermore, it defines the element during a specific mixing. The solution mixture is frequently defined in terms of mass percentage. It represents the amount of solute contained in a mass m of solution.
The quantity of solutes can be stated in terms of mass or moles. We will study the percent by mass formula with numerous solved numerical examples in this post.
Mass percent = (component’s mass ÷ total mass) x 100%
= (1/ 23.95)x 100%
= 41.7%
Therefore, 41.7% is the percentage by mass of hydrogen (H) in lithium hydroxide (LiOH).
To learn more about percentage by mass, here:
https://brainly.com/question/28998211
#SPJ1
which phrase explains the apparent motions of planets in the night sky?
Answers
The east to west daily motions of stars, planets, the Moon, and the Sun are caused by the rotation of the Earth on its axis. The Earth and all the planets revolve around the Sun on circular orbits. This produces the change in constellations observed from one time of year to the next.
hope it helps..!!!