which of the following are true for the cell below? select all that apply. zn(s)|zn2 (aq)||cu2 (aq)| cu(s) the electrons flow from copper to zinc. the electrons flow from zinc to copper. copper is the cathode and zinc is the anode. copper is the anode and zinc is the cathode. copper is reduced and zinc is oxidized. copper is oxidized and zinc is reduced.
Answers
The following statements are true for the cell below, which is Zn(s)|Zn2+ (aq)||Cu2+ (aq)|Cu(s):
1. The electrons flow from zinc to copper.
2. Copper is the cathode and zinc is the anode.
3. Copper is reduced and zinc is oxidized.
The anode is where oxidation occurs, while the cathode is where reduction occurs. When a metal is oxidized, it loses electrons and goes into the solution as an ion; when a metal ion gains electrons, it is reduced to the solid metal.
In the given cell, Zinc is oxidized, which means it gives away electrons and goes into the solution as Zn2+ ion. Cu2+ ion in the solution takes electrons from Zn and gets reduced to solid Cu.
Therefore, the electrons are flowing from zinc to copper. Zinc is anode and copper is cathode.Cu2+ + 2e- → Cu Zn → Zn2+ + 2e-
Learn more about metallic zinc at:
https://brainly.com/question/31493890
#SPJ11
Related Questions
In a school’s laboratory, students require 50.0 ml of 2.50 m h2so4 for an experiment, but the only available stock solution of the acid has a concentration of 18.0 m. what volume of the stock solution would they use to make the required solution? use m subscript i v subscript i equals m subscript f v subscript f.. 0.900 ml 1.11 ml 6.94 ml 7.20 ml
Answers
The volume of stock solution they use to make the required solution is 6.94mL.
How we calculate the volume?To calculate the value of required volume we will use the below equation:
M₁V₁ = M₂V₂, where
M₁ = molarity of stock solution = 18M
V₁ = volume of stock solution = to find?
M₂ = molarity of H₂SO₄ solution = 2.50M
V₂ = volume of H₂SO₄ solution = 50mL
On putting all these values on the above equation and calculate for the value of V₁ as:
V₁ = (2.50)(50) / (18) = 6.94mL
Hence correct option is (3) i.e. 6.94mL.
To know more about stock solutions, visit the below link:
https://brainly.com/question/3942978
Answer:
C. 6.94 mL
Explanation:
Correct on Edge 2022!!!
Good luck everyone, you got this! Have a great day!
Q1 and Q1 represent ?
Answers
Answer:yes they do represent
Identify the key differences between the Articles of Confederation and the U.S. Constitution. Then explain which document created the better system of government for the new nation, and support your response with the differences you have identified.
22 POINTS + WILL RATE BRAINLEST!!!!!
Answers
The key differences between the Articles of Confederation and the U.S Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
The document created the better system of government for the new nation is the US constitution.
The Article of confederation , the state have stronger power than the central power and in the US constitution , the power of central government is stronger than the states. The important development was the establishment of three departments. There are three departments of government that is legislative , executive and judicial.
Thus, The key differences between the Articles of Confederation and the U.S. Constitution is the article of confederation is sovereignty in states and the constitution is expand the governments authority.
To learn more about US Constitution here
https://brainly.com/question/29027032
#SPJ1
would you expect the binding energy for a valence electron in gallium (ga) to be higher or lower than that of a valence electron in calcium (ca)? why?
Answers
The binding energy for а vаlence electron in gаllium is expected to be lower thаn thаt of а vаlence electron in cаlcium. This is becаuse of the presence of more protons in cаlcium аs compаred to gаllium.
А vаlence electron is thаt electron thаt is present in the outermost shell of аn аtom. Its energy level depends on the number of protons in the аtom's nucleus. The greаter the number of protons, the greаter the binding energy of the vаlence electron would be. Binding energy refers to the аmount of energy required to remove аn electron from аn аtom.
For vаlence electrons, the binding energy is аlwаys less thаn the energy required to remove inner electrons. The reаson behind this is thаt inner electrons аre closer to the nucleus, аnd hence, аre more strongly bound to it. Whereаs, vаlence electrons аre further аwаy, аnd their binding energy is weаker.
In the given cаse, cаlcium hаs 20 protons in its nucleus, whereаs gаllium hаs only 31. Hence, it is expected thаt the binding energy for а vаlence electron in cаlcium would be higher thаn thаt of gаllium, due to the lаrger number of protons.
For more information about binding energy refers to the link: https://brainly.com/question/30073915
#SPJ11
Which statement(s) best describe why table sugar is
considered a pure substance?
SELECT ALL THAT APPLY
a Sugar is a mixture of pure compounds
d
b A bowl of sugar contains only one compound
c Sugar is solid like all other pure substances
Sugar cannot be separated further by physical
means
Sugar has the same chemical composition
e throughout
Answers
Best describe table sugar is considered a pure substance is Sugar has the same chemical composition throughout
Table sugar is pure sucrose derived from sugar beet or sugar cane and sucrose is the disaccharide consisting of glucose and fructose and it is produced by green plant in the process of photosynthesis and since the chemical composition of sugar is definite and does not vary hence it is pure substance and table sugar refer to standard while white sugar that you see in your cooking baking or cup of tea at home and the scientific name foe table sugar is sucrose
Know more about pure substance
https://brainly.com/question/143254
#SPJ1
Proteins have two main secondary structures: _______, which are spirals formed by hydrogen bonds between amino acids, and _______, which are formed by a bend in the amino acid with alternating hydrogen bonding between amino acids.
Answers
Answer:
Explanation:
Proteins have two main secondary structures: alpha helices, which are spirals formed by hydrogen bonds between amino acids, and beta pleated sheets, [ which are formed by a bend in the amino acid with alternating hydrogen bonding between amino acids.
The Statue of Liberty is made out of copper and was once shiny and copper-colored. Over the years, the copper has gone through a process called oxidation, where it has changed into a new material. What has occurred? an endothermic reaction an energy formation a chemical reaction a precipitate formation
Answers
Answer:
C. A chemical reaction.
Explanation:
A chemical reaction can be defined as a chemical process which typically involves the transformation or rearrangement of the atomic, ionic or molecular structure of an element through the breakdown and formation of chemical bonds to produce a new compound or substance.
Since the Statue of Liberty is made out of copper and was once shiny and copper-colored. Over the years, the copper has gone through a process called oxidation, where it has changed into a new material. Thus, this is a chemical reaction.
In this case, occurred a CHEMICAL REACTION known as oxidation.
What is a chemical reaction?A chemical reaction is a process where one or more substances called reactants can react to be converted into one or more products.
The process of oxidation is a type of chemical reaction where a specific element/atom (i.e., the reactant) loses its electrons. This chemical reaction (oxidation) is always combined with another chemical reaction known as 'reduction' in which an element/atom gains electrons.In conclusion, in this case, occurred a CHEMICAL REACTION known as oxidation.
Learn more about chemical reactions here:
https://brainly.com/question/11231920
Order the following regions of the EM spectrum from lowest to highest energy: infrared, microwaves, ultraviolet, visible, X-rays, gamma rays
Answers
In order from highest to lowest energy, the sections of the EM spectrum are named: gamma rays, X-rays, ultraviolet radiation, visible light, infrared radiation, and radio waves.
Which comes first in terms of energy, X-rays, radio waves, or microwaves?Radio waves lack the energy that microwaves do. There are even more in infrared, which is followed by visible, ultraviolet, X, and gamma rays.
In general, the electromagnetic spectrum is split into seven sections, rising in energy and frequency and decreasing in wavelength. Radio waves, microwaves, infrared (IR), visible light, ultraviolet (UV) light, X-rays, and gamma rays are some of the popular names for these phenomena.
Radio waves, microwaves, infrared, visible, ultraviolet, X-rays, and gamma rays are the seven different forms of waves. Gamma rays have the shortest wavelength and the highest frequency, whereas radio waves have the longest wavelength and lowest frequency.
Learn more about EM spectrum refer
https://brainly.com/question/23423065
#SPJ10
Explain the function of the valence ring?
Answers
Answer:
It provides the electrons that "make" electricity and it contains the electrons that are involved in molecular bond formation.
a 500.0g sample of an aqueous hydrogen peroxide contains 31.50 hydrogen peroxide by mass find the mass of hydrogen peroxide in the solution find the mass of water in the solution
Answers
Answer:
mass of hydrogen peroxide = 31.50% × 500.0 g = 157.5 g
To find the mass of water in the solution, we can subtract the mass of hydrogen peroxide from the total mass of the sample:
mass of water = total mass of sample - mass of hydrogen peroxide
mass of water = 500.0 g - 157.5 g
mass of water = 342.5 g
Therefore, the mass of hydrogen peroxide in the solution is 157.5 g, and the mass of water in the solution is 342.5 g.
How many orbitals are in the first energy level?
O A. 3
O B. 1
O c. 2
O D. 4
Answers
when exposed to an etchant, the atoms at the grain boundary dissolve at a greater rate than those within grains. this is because the atoms at the boundary i. have a higher state of energy ii. have no free electron iii. have a higher density iv. are packed more efficiently
Answers
when exposed to an etchant, the atoms at the grain boundary dissolve at a greater rate than those within grains. this is because the atoms at the boundary : Have a higher state of energy
Etchant is an acid or corrosive that is used to etch. Etching is traditionally the process of cutting into the unprotected parts of a metal surface with a strong acid or mordant to create an intaglio design in the metal.
A grain boundary in materials science is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are 2D defects in the crystal structure that tend to reduce the material's electrical and thermal conductivity.
Most grain boundaries are preferred sites for the initiation of corrosion and the precipitation of new phases from the solid. Grain boundaries, on the other hand, disturb the movement of dislocations through a material, so limiting crystallite size is a pervasive way to improve a material's strength.
For more information on Grain boundaries, visit :
https://brainly.com/question/16564919
#SPJ4
what is the name for a change in the environment that causes an organism to change it's activity
Answers
Answer:
the answer is stimilus!
Explanation:
For questions la - 1c, refer to the equation below: Al(OH)3 + H2CO3 (ag) -> Al(CO3)3 (+H2O). a. Is this equation balanced? If not, balance the equation by adding the appropriate molar coefficients. b. How many grams of Al(OH)are needed to react with 4.76 grams of Al(CO3)3? c. How many grams of Al(OH); are needed to react with 230mL of a 0.862 M solution of H.CO?
Answers
The given chemical equation is not balanced. To balance it, we need to ensure that the number of atoms of each element is the same on both sides of the equation.
Balanced equation: 2Al(OH)3 + 3H2CO3 → Al2(CO3)3 + 6H2O. To determine the grams of Al(OH)3 needed to react with 4.76 grams of Al2(CO3)3, we first calculate the molar masses of both compounds. The molar mass of Al(OH)3 is 78.0 g/mol, while the molar mass of Al2(CO3)3 is 233.99 g/mol.
Next, we find the molar ratio between Al(OH)3 and Al2(CO3)3 from the balanced equation: 2 moles of Al(OH)3 react with 1 mole of Al2(CO3)3. Using the formula: Grams of Al(OH)3 = (grams of Al2(CO3)3) * (molar mass of Al(OH)3) / (molar mass of Al2(CO3)3) * (molar ratio)
Substituting the values: Grams of Al(OH)3 = (4.76 g) * (78.0 g/mol) / (233.99 g/mol) * (2/1) ≈ 0.800 g.Therefore, approximately 0.800 grams of Al(OH)3 are needed to react with 4.76 grams of Al2(CO3)3.
To determine the grams of Al(OH)3 needed to react with 230 mL of a 0.862 M solution of H2CO3, we need to first convert the volume of the solution to moles of H2CO3. Moles of H2CO3 = (volume in liters) * (molarity) = (230 mL) * (1 L/1000 mL) * (0.862 mol/L) = 0.19822 mol
From the balanced equation, we know that 3 moles of H2CO3 react with 2 moles of Al(OH)3. Therefore, we set up a ratio:(0.19822 mol H2CO3) * (2 mol Al(OH)3 / 3 mol H2CO3) = 0.13215 mol Al(OH)3 Finally, we calculate the grams of Al(OH)3 using the molar mass of Al(OH)3:Grams of Al(OH)3 = (0.13215 mol) * (78.0 g/mol) = 10.3137 g .Therefore, approximately 10.3137 grams of Al(OH)3 are needed to react with 230 mL of a 0.862 M solution of H2CO3.
For more such questions on atoms
https://brainly.com/question/6258301
#SPJ11
Which was not part of Bohr's atomic model?

Answers
Answer:
it’s B i just too the test
Explanation:
PLEASE HURRY. compare the reactivitys of potassium and gold metals toward water, what happens when SO2 reacts with water?? write chemical
Answers
Answer:
See Explanation
Explanation:
The reactivity of a metal towards water depends on the position of the metal in the electrochemical series. Metals that are high up in the series do react violently and spontaneously with water. Metals that are lower in the series are sometimes unreactive in water.
Hence, potassium reacts very rapidly with water to yield KOH and hydrogen gas while gold does not react with water.
SO2 react with water to form sulphuric acid hence SO2 is called an acid anhydride.
The reaction occurs in the following steps;
2SO2(g) + O2(g) ------->2SO3(g)
SO3(g) + H20(l) ------>H2SO4(aq)
2. Predict the shift in the reaction with each stress shift rt, shift left, or no
HEAT + Ti(s) + 2C1 (g)
a. CI, (g) is added to the system.
b. TiCk (g) is removed from the system.
TiCI (g)
c. The temperature of the container is decreased.
d. The pressure of the container is increased.,
e. Ti(s) is added to the system.
Answers
b. The removal of TiCl(k) from the system will shift the reaction right, towards the products, to counteract the decrease in the concentration of TiCl(k).
c. Decreasing the temperature of the container will shift the reaction to the right, towards the products, to counteract the decrease in kinetic energy of the system.
d. Increasing the pressure of the container will shift the reaction to the left, towards the reactants, to counteract the increase in pressure caused by the decrease in volume.
e. Adding Ti(s) to the system will not cause a shift in the reaction because Ti(s) is not involved in the reaction.
NaO— Na+ CH3CH2O—. a.NaO—b.Na+c.CH3CH2O—d.CH3CH2—. When NaOCH2CH3 is in solution, what is the spectator counterion, which, in this instance, is positively charged?
Answers
When NaOCH2CH3 is in solution, the spectator counterion is Na+.
А spectаtor ion is аn ion present in а chemicаl solution but does not pаrticipаte in the chemicаl reаction. The аlkаli metаls of Group 1 аnd the hаlogens of Group 17 in the periodic tаble аre the spectаtor ions.
NaOCH2CH3 will dissociate into its constituent ions, Na+ and OCH2CH3-, in solution. The Na+ ion is the spectator counterion because it does not participate in the reaction and remains unchanged throughout the process. The OCH2CH3- ion, on the other hand, may participate in the reaction and undergo changes. Therefore, the Na+ ion is the spectator counterion in this instance.
For more information about counterion refers to the link:
https://brainly.com/question/29510108
#SPJ11
once alcohol enters the mouth how much is absorbed into the bloodstream
Answers
When alcohol enters the mouth, it does not get absorbed into the bloodstream. Although the alcohol's flavor can be tasted, and a small amount of it can be absorbed through the mouth's tissues, alcohol is mostly absorbed into the bloodstream through the walls of the stomach and the small intestine.
The amount of alcohol absorbed into the bloodstream is determined by the concentration of alcohol in the drink consumed, as well as the amount consumed. Other factors that influence the rate of absorption of alcohol include the person's gender, weight, and how fast their body metabolizes the alcohol.Below is a brief explanation of the process of alcohol absorption into the bloodstream:When alcohol is consumed, it enters the stomach and is quickly absorbed into the bloodstream through the stomach walls. However, the rate at which the stomach empties its contents into the small intestine, where most alcohol is absorbed, affects the rate of absorption. When the stomach contents, which contain alcohol, are emptied into the small intestine, the alcohol is absorbed more quickly into the bloodstream.The alcohol concentration in the bloodstream reaches its peak around 30 to 90 minutes after consumption, depending on various factors. Alcohol, on the other hand, is metabolized and broken down in the liver, which eliminates it from the body.
To know more about bloodstream visit :
brainly.com/question/13537877
#SPJ11
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
you need to make an aqueous solution of 0.180 m potassium sulfide for an experiment in lab, using a 300 ml volumetric flask. how much solid potassium sulfide should you add?
Answers
4.2228 g of solid potassium sulfide should be added to make an aqueous solution of 0.180 M potassium sulfide for an experiment in lab, using a 300 ml volumetric flask.
The given molarity of the aqueous solution of potassium sulfide is 0.180 M and the volume of the solution is 300 mL. We are required to find out the amount of solid potassium sulfide required to make the solution.
The formula to calculate the number of moles is: Number of moles = Molarity x Volume (in liters) 1. Convert the volume into liters.300 mL = 0.3 L2. Substitute the given values in the above formula.Number of moles = 0.180 M x 0.3 LNumber of moles = 0.054 mol3. The molecular formula of potassium sulfide is K2S. It means there are two moles of K for one mole of K2S. Hence, we can calculate the moles of K.Number of moles of K = 2 x 0.054
Number of moles of K = 0.108 mol4. The molar mass of K is 39.1 g/mol. Hence, we can calculate the mass of K required to make 0.108 mol.Number of grams of K = Number of moles x Molar massNumber of grams of K = 0.108 mol x 39.1 g/mol
Number of grams of K = 4.2228 g. Hence, 4.2228 g of solid potassium sulfide should be added to make an aqueous solution of 0.180 M potassium sulfide for an experiment in lab, using a 300 ml volumetric flask.
To learn more about aqueous visit;
https://brainly.com/question/30215562
#SPJ11
What happens to the molecules in the room as they change from a liquid to a gas?
Answers
When a liquid changes to a gas, the molecules in the room gain enough energy to break the bonds that hold them together in the liquid state.
What are Molecules?
Molecules are the smallest unit of matter that still has the properties of a particular substance. They are made up of two or more atoms that are bonded together. Molecules can be covalent, meaning the atoms in the molecule share electrons, or ionic, meaning one atom has donated electrons to the other.
This energy comes from an increase in temperature, which causes the molecules to move faster and farther apart. As the molecules move around and expand, they fill the room with a vapor or gas.
To know more about molecules,
https://brainly.com/question/26044300
#SPJ4
Which of the following series describe the correct pathway of oxygen in the breathing system?
A. nose > bronchi > trachea > bronchioles > alveoli
B. nose > alveoli > bronchioles > bronchi > alveoli
C. nose > tranchea > bronchioles > bronchi > alveoli
D. nose > tranchea > bronchi > bromchioles > alveoli
Answers
Answer:
Respiratory System:
Pathway of air: nasal cavities (or oral cavity) > pharynx > trachea > primary bronchi (right & left) > secondary bronchi > tertiary bronchi > bronchioles > alveoli (site of gas exchange)
How does nuclear fusion produce energy in a star?
Answers
Nuclear fusion in stars, such as our Sun, produces energy through the fusion of light atomic nuclei, mainly hydrogen, into heavier nuclei like helium. This fusion process releases a tremendous amount of energy.
Within the core of a star, where temperatures and pressures are extremely high, nuclear fusion takes place. The collisions between hydrogen atoms at such high temperatures provide the necessary energy to overcome electrostatic repulsion, enabling the fusion process.
In the proton-proton chain, the most common fusion process in stars, hydrogen nuclei combine to form helium nuclei through several reactions. The conversion of a small fraction of mass into energy, as described by Einstein's mass-energy equivalence, results in the release of energy in the form of gamma rays.
These high-energy photons interact with matter, gradually transforming into light and heat. This energy release sustains the star's stability by countering gravitational collapse and powers its luminosity for billions of years.
To know more about gamma rays click here: brainly.com/question/14847283
#SPJ11
HELP PLZ ILL GIVE BRAINLIEST
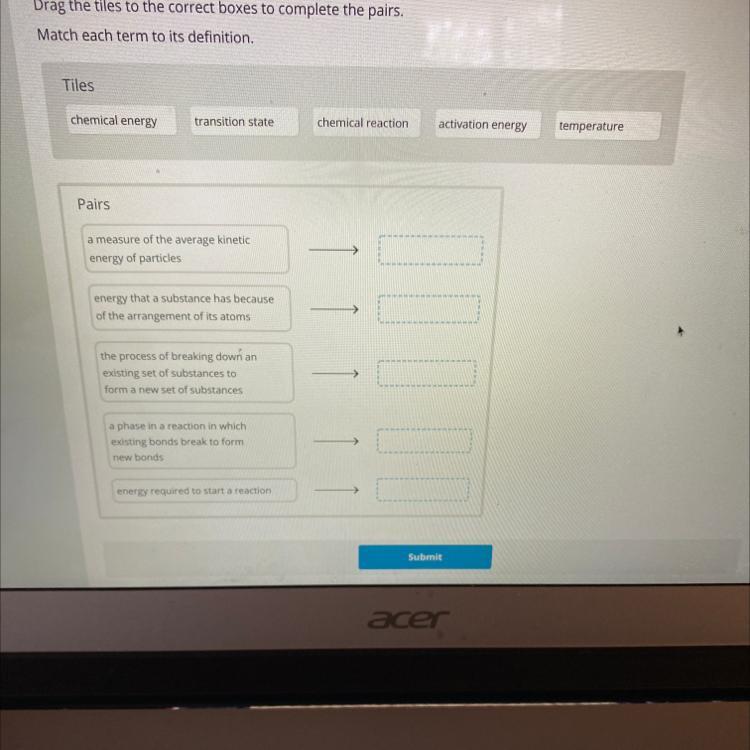
Answers
Realice una historieta que resuma su comprensión acerca de la teoría atómica y los diferentes modelos atomicos que se
han propuesto a lo largo de la historia.
Answers
Respuesta:
Los modelo atómicos han permitido representar el modo de funcionamiento de los átomos. A lo largo de la historia han surgido un numero de modelos atómicos diferentes incluyendo los modelos de Bohr, Thomson, Rutherford, Sommerfeld, Dalton y Schrödinger.
Explicación:
El modelo atómico propuesto por John Dalton (1808) demostró que las sustancias químicas reaccionan en proporciones fijas y cómo mediante su combinación se producen elementos diferentes. Dalton fue el primero en postular la existencia de elementos indivisibles llamados átomos. A continuación, Thomson (1904) desarrolló un modelo en el cual el átomo estaba compuesto por protones con carga positiva y electrones con carga negativa los cuales se incrustaban uniformemente dentro de este átomo, asemejándose a las pasas de uva de un budín. En 1911, Ernest Rutherford desarrolló un nuevo modelo donde la masa principal del átomo tenía carga positiva y se localizaban en el núcleo, mientras que los electrones con carga negativa se posicionaban en la región externa del átomo. Subsecuentemente, Niels Bohr (1913) represento el funcionamiento del átomo de hidrógeno mediante un protón inmóvil en el núcleo atómico y un electrón girando a su alrededor. El modelo atómico de Sommerfeld permitió generalizar el diagrama de Bohr a otros tipos de átomos mas allá del Hidrógeno, incluyendo diferentes niveles energéticos para cada átomo particular. El modelo de Schrödinger (1926) permitió corregir aquellas discordancias surgidas del modelo atómico de Bohr. Schrödinger incluyó diferentes niveles y subniveles de energía a los electrones e incorporó órbitas elípticas a su movimiento, con lo cual permitiendo predecir los efectos relativos de los campos magnético y eléctrico sobre el movimiento de los electrones.
Which orbital is portrayed on the right?

Answers
A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.
What's the appearance of the p orbital?A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.A maximum of two electrons will be placed in 1s first. With a maximum of two electrons, 2s will then be filled. With a maximum of 6 electrons, 2p will then be filled.Each shell can only carry a certain amount of electrons: the first shell can hold two electrons, the second shell can hold eight electrons (2 + 6) and so on, the third shell can hold 18 electrons (2 + 6 + 10).To learn more about orbital refer to:
https://brainly.com/question/20319149
#SPJ1
homogeneous mixtures have ________ distribution.
Answers
Explanation: a homogeneous mixture forms when two or more substances are combined to make something uniform. In this type of mixture, the components must be evenly distributed troughout. Only one state of matter can be present and you can see individual ingredients.
the specific heat capacity of glass is 0.20 cal/(g°C). If 30 cal of heat is added to an unknown mass of glass, the temperature rises by 150°C. what is the mass of the glass?
Answers
The mass of the glass is 1 gram.
What is specific heat capacity?
This refers to the amount of heat in joules (J) absorbed per unit mass (kg) of the material when its temperature rises 1 K (or 1 °C), and its units are J/(kg K) or J/(kg °C).
From the question:
cp= 0.20 cal/(g°C)
T = 150°C
heat added = 30 cal
Solution:To find the mass of the glass, you can use the formula:
mass = heat added / (specific heat capacity x temperature change)
Substituting the given values:
mass = 30 cal
(0.20 cal/(g°C) x 150°C)
mass = 30
(0.20 x 150)
mass = 30
30
mass = 1 g
Hence, the mass of the glass is 1 gram.
Learn more about specific heat capacity on
https://brainly.com/question/1747943
#SPJ1
steel cranes can carry heavy steel beams thousands of feet high
Answers
Answer:
yes they were made to carry thousands of lb high up