Answers
The monoatomic ion of potassium is K+ and Oxygen is O2-
The term "monatomic ion" refers to an ion with only one atom. A polyatomic ion is one that has more than one atom, even if they are all from the same element.The monatomic ions generated by potassium (K) and Oxygen (O) are being askedwe will first look for the group number that an element belongs to in order to establish the charge of that element. For non-metals, the charge of the ion is calculated by deducting 8 from the group number For metals, the charge of the ion is equal to the group number.Since K is a metal, its ion is a cation with a charge of 1, and it is a member of group 1A. K+ is hence the potassium's monoatomic ion.similarly oxygen is non metal therefore the monoatomic ion of oxygen is O2−.Learn more about monoatomic ions here:
https://brainly.com/question/13746513
#SPJ9
Related Questions
Q1- Classify each of the molecules given below by molecular geometry.
1- Sulfur Hexafluoride (SF6)
2-Methane (CH)
3- Carbon dioxide (CO2)
4-Boron Trifluoride (BF3)
5-Sulfur Trioxide (SO3)
6-Carbon Tetrachloride (CC1.),
7-Phosphorus Pentachloride (PC15)
8-Beryllium Chloride (BeCl2)
Answers
A researcher observes a reaction and gathers the data in the table below. Observations Mass decreased after reaction Energy is released during reaction New substance is formed Which piece of evidence best identifies they type of reaction as nuclear or chemical? 1. Chemical, because energy is released during the reaction. 2.Nuclear, because energy is released during the reaction. 3.Nuclear, because the mass decreased after the reaction. 4.Chemical, because a new substance is formed.
Answers
The piece of evidence that best identifies the type of reaction as nuclear or chemical is: Chemical, because a new substance is formed. Option 4
In this scenario, the observation that a new substance is formed is a key characteristic of a chemical reaction. Chemical reactions involve the rearrangement of atoms to form different substances with distinct properties. The formation of a new substance indicates a chemical change has occurred.
The other pieces of evidence listed do not necessarily point to a nuclear reaction:
Chemical, because energy is released during the reaction: Energy can be released in both nuclear and chemical reactions, so this observation alone is not sufficient to determine the type of reaction.
Nuclear, because energy is released during the reaction: While energy can be released in nuclear reactions, it is not exclusive to them. Chemical reactions can also release energy, such as in exothermic reactions.
Nuclear, because the mass decreased after the reaction: This observation suggests a change in mass, which could be indicative of a nuclear reaction. However, it is important to consider that chemical reactions can also involve changes in mass, such as the formation of gases or dissolution of a solid.
Overall, the most conclusive evidence to identify the type of reaction is the formation of a new substance, which aligns with a chemical reaction.
Option 4
For more such questions on Chemical visit:
https://brainly.com/question/29886197
#SPJ8
why liquid pressure increases with depth
Answers
Answer:
Pressure increases as the depth increases. The pressure in a liquid is due to the weight of the column of water above. Since the particles in a liquid are tightly packed, this pressure acts in all directions. For example, the pressure acting on a dam at the bottom of a reservoir is greater than the pressure acting near the top.
Explanation:
Which of the following statements is true?
A.
Chemical reactions can either absorb thermal energy or release thermal energy.
B.
Chemical reactions can only release thermal energy.
C.
Chemical reactions can only absorb thermal energy.
D.
Chemical reactions can neither absorb thermal energy nor release thermal energy.
Answers
Ionic compounds do not conduct electricity in the solid state, but do conduct electricity in what 2 states
Answers
Ionic compounds conduct electricity in an aqueous solution and molten state but do not conduct electricity in a solid state.
What are ionic compounds?Ionic compounds can be defined as crystalline solids generated by closely packed ions with opposite charges. An Ionic compound can be described as generally formed when one metal reacts with a non-metal.
In ionic compounds, the ions in the compound are usually held together by ionic bonds. The ions are produced by gaining or losing electrons in order to attain the nearest noble gas configuration.
In a reaction, the metals commonly lose electrons to achieve their complete octet while non-metals gain electrons to get a full octet.
In the solid state, Ionic compounds do not conduct electricity because ions are at fixed positions and do not move.
Learn more about ionic compounds, here:
brainly.com/question/17217225
#SPJ1
Suppose a solution has a density of 1.87 g/mL. If a sample has a mass of 17.5 g the volume of the sample in mL is what?
Answers
We can use the formula:
Density = Mass/Volume
Rearranging the formula gives:
Volume = Mass/Density
Substituting the given values gives:
Volume = 17.5 g / 1.87 g/mL = 9.36 mL.
If an experiment produces 5 g but should have made 500 g, what is the percent
yield?
Answers
Answer:
Percentage of yield = 1%
Explanation:
Given:
Amount of yield = 5g
Total amount of product = 500 gram
Find:
Percentage of yield = ?
Computation:
⇒ Percentage of yield = [Amount of yield / Total amount of product]100
⇒ Percentage of yield = [5g / 500g]100
⇒ Percentage of yield = [0.01]100
⇒ Percentage of yield = 1%
helpp pleasee.............

Answers
Answer:
it's answer is lithium and boron.
When ATP breaks down to ADP, potential energy stored in bonds is released. This energy stored in bonds is
Answers
I don't know what category to put this question in, but I attached a photo of it. Can someone please help me answer it?
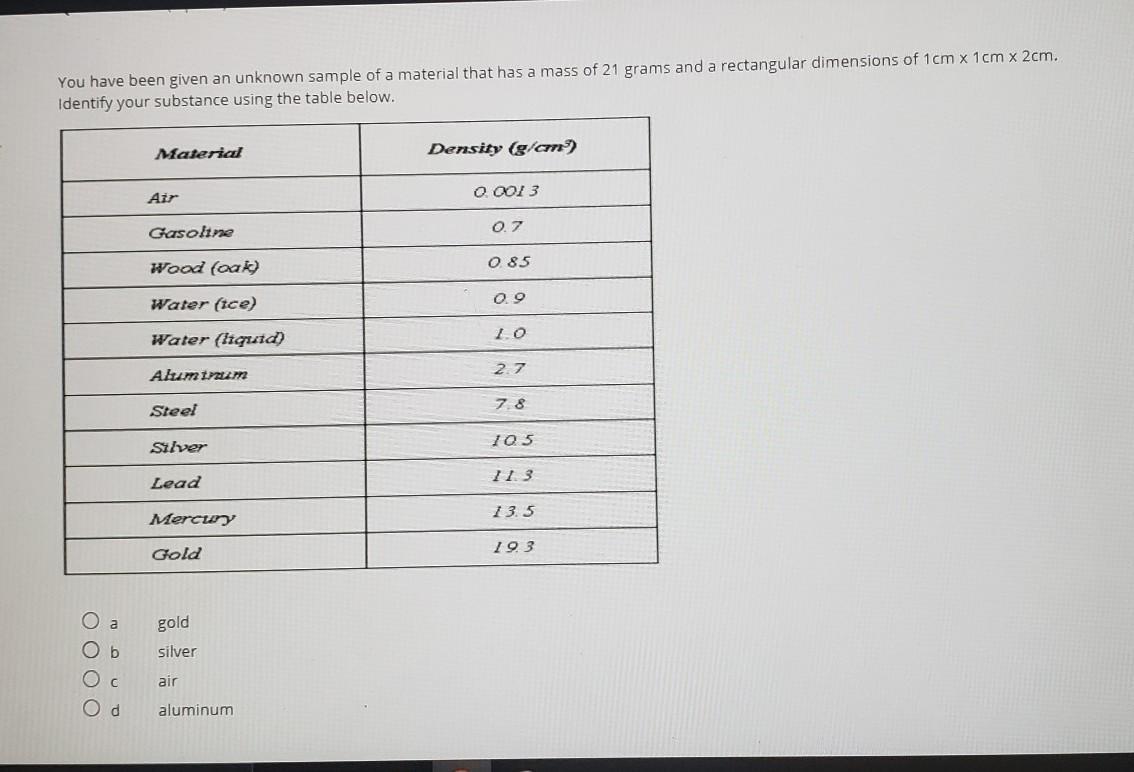
Answers
Analysing the question:
To calculate the density of a material, we need its mass and volume
We are given:
Mass of sample = 21 grams
dimensions of the sample = 1 * 1 * 2 = 2 cm³
Calculating the density:
Density = Mass of sample / volume of sample
Replacing the variables
Density = 21 / 2
Density = 10.5 g / cm³
Determining the Material:
From the table provided, we can see that the density of Silver is 10.5 g/cm³
Therefore, the material is Silver
1. Which of the following is NOT Matter?
chair
air
light
water
Answers
Describe briefly how to obtain the radial probability of an electron
Answers
Explanation:
The radial distribution function gives the probability density for an electron to be found anywhere on the surface of a sphere located a distance r from the proton. Since the area of a spherical surface is 4πr2, the radial distribution function is given by 4πr2R(r)∗R(r).
I
Use the following balanced reaction to solve 1-3:
P4 (s) + 6H2 (g) → 4PH3 (g)
How many grams of phosphorus trihydride will be formed by reacting 60 L of Hydrogen gas with an excess of P4?
Answers
Answer:
60.86 g of PH₃
Explanation:
We'll begin by calculating the number of mole of H₂ that will occupy 60 L. This can be obtained as follow:
22.4 L = 1 mole of H₂
Therefore,
60 L = 60 / 22.4
60 L = 2.68 mole of H₂.
Next, we shall determine the number of mole of PH₃ produced by the reaction of 60 L (i.e 2.68 mole) of H₂. This can be obtained as follow:
P₄ + 6H₂ –> 4PH₃
From the balanced equation above,
6 moles of H₂ reacted to produce 4 moles of PH₃.
Therefore, 2.68 moles of H₂ will react to to produce = (2.68 × 4)/6 = 1.79 moles of PH₃.
Finally, we shall determine the mass of 1.79 moles of PH₃. This can be obtained as follow:
Mole of PH₃ = 1.79 moles
Molar mass of PH₃ = 31 + (3×1)
= 31 + 3 = 34 g/mol
Mass of PH₃ =?
Mass = mole × molar mass
Mass of PH₃ = 1.79 × 34
Mass of PH₃ = 60.86 g
Thus, 60.86 g of PH₃ were obtained from the reaction.
An imaginary line dividing the earth's surface into two hemisphere the northern and southern hemisphere, it is locatedat 0⁰, which of the following imaginary lines is being described? a.equator b.latitude c.longitude d.prime meridian
Answers
Answer:
A. Equator
Explanation:
The equator is located in the centre of the Earth, dividing the northern and southern hemispheres.
Answer:
Equator because equator divides the earth into Northern and Southern hemisphere.
The answer to this question

Answers
1) There are only single bonds between the carbon atoms, so the ending is 'ane'. Thus, the answer is 2-methylbutane.
2) There is one triple bond between one pair of carbon atoms, so the ending is 'yne'. So, the answer is 2-butyne.
3) There is one double bond between one pair of carbon atoms, so the ending is 'ene'. So, the answer is 2-butene.
How can you use the periodic table to predict reactivity?
Giving Brainliest!!!
Answers
Answer:
hi
Explanation:
how r u today
If a molecule has a triple bond, what can be assumed about the bond compared to a molecule with a double bond?
The length is more than a double bond
The strength is more than a double bond
The strength is less than a double bond
The length is the same as a double bond
Answers
While the length is less than a double bond, the strength exceeds that of a double bond.
Within the same molecule, how do triple bonds differ from double bonds?Due to the presence of two bonds rather than one, triple bonds are stronger than double bonds. An sp-sp sigma bond is created when one of each carbon atom's two sp hybrid orbitals intersects with the corresponding orbital from the other carbon atom.
Compared to double bonds, are triple bonds more durable and longer?Six electrons are shared by a sigma bond, two bonds, and a triple bond. Double bonds are more powerful than single bonds, and triple bonds are more powerful than double bonds, according to experiments.
To know more about double bond visit:-
https://brainly.com/question/30575593
#SPJ1
Answer: third
Explanation:
if anybody could help with this question i would appreciate it
its not college level but i dont know how to change the grade level

Answers
Answer:
D. Liquid
Explanation:
It's mercury given the density and mercury's a metal that is liquid at room temperature
please can someone help me out with this? i need it very quickly need answer in 4 hours pls help

Answers
Explanation:nthere is your work

If you have 600g of nitroglycerin, how many moles do you have?
help please
Answers
Answer:
600
Explanation:
there's 1 mole in every nitroglycerin
I think
What is the total number of moles of O2(g) that must react completely with 5.00 moles of C4H10(g)?
Answers
Answer:
32.5 moles of O2(g) are necessaries for a complete reaction
Explanation:
Based on the reaction of combustion of C4H10(g):
C4H10(g) + 13/2 O2(g) → 4CO2(g) + 5H2O(g)
1 mole of C4H10 needs 13/2 moles O2 for a complete reaction.
That means, the moles of O2 required for a reaction of 5.00 moles of C4H10 are:
5.00 moles C4H10 * (13/2mol O2 / 1mol C4H10) =
32.5 moles of O2(g) are necessaries for a complete reaction
how is the Bohr atomic model different from the plum-pudding model ?
Answers
The plum-pudding model suggested a uniform distribution of electrons within a positively charged atom, whereas the Bohr atomic model introduced the concept of quantized energy levels and specific orbits for electrons.
The Bohr atomic model and the plum-pudding model are two distinct models that were proposed to explain the structure of atoms, and they differ in their fundamental concepts.
The plum-pudding model, also known as the Thomson model, was proposed by J.J. Thomson in 1904. According to this model, an atom consists of a positively charged sphere (the "pudding") with embedded negatively charged electrons (the "plums").
In other words, the electrons were thought to be uniformly distributed throughout the positively charged atom. This model suggested that the atom was overall neutral and did not contain any distinct substructures.
On the other hand, the Bohr atomic model, proposed by Niels Bohr in 1913, introduced the concept of quantized energy levels within an atom. According to this model, electrons orbit the nucleus in specific, discrete energy levels or shells.
These energy levels are represented by fixed orbits or paths, with electrons occupying only certain allowed orbits. The model also introduced the idea that electrons can transition between energy levels by emitting or absorbing energy in discrete packets called photons. This model explained phenomena like atomic spectra and the stability of atoms.
For mopre such questions on Bohr atomic model visit:
https://brainly.com/question/18002213
#SPJ8
The wind causes this wind turbine to spin, creating electricity. The moving blades are evidence of A) solar power. B) work being done. C) potential energy. D) mechanical advantage.
Answers
Can two different isotopes of the same element ever have the same atomic mass?
Answers
No, it's not possible.
Isotope means two atoms which have same proton number but varying neutron number. And we calculate mass as the summation of neutron & proton number. If the proton number varies, mass will also vary.
PLEASE HELP!! 30 PTS
Identify the combination reaction.
1.) 2H 2 + O 2 ⟶ 2H 2O
2.) Cl 2 + 2KBr ⟶ 2KCl + Br 2
3.) Al 2S 3 ⟶ 2Al + 3S
4.) C 4H 12 + 7O 2 ⟶ 6H 2O + 4CO 2
Answers
Answer:
1.) 2H2 + O2=2H2O
2.)Cl2 + 2KBr= 2KCl + Br2
4.)C4H12 + 7O2= 6H2O + 4 CO2
Plz help me ASAP in my final project I am ready to pay 20$
Answers
Answer:
what do you need help with
Mass and height are _______ proportional to potential energy.
Question 4 options:
directly
indirectly
exponentially
not
Answers
Answer:
directly
Explanation:
click the brainiest answer and please thank me too
1 mole of sulfur atoms has how much mass
Answers
Answer:
One atom of sulfur has a mass of 32.07 AMU; one mole of S atoms has a mass of 32.07 g.
Explanation:
Therefore, the answer should be 32.07 g
What shell contains a total of 9 orbitals?
Answers
Answer:
Explanation:
The shell number 3 can contain 9 (and more) electrons.
The shell number 2 can contain a maximum of 8 electrons.
There are 4 different types of orbitals: s, p, d and f. So, the maximum number of orbitals that a shell can con

2KClO3-2KCl+3O2 how many moles of O2 can be produced by decomposing 12 moles of KClO3?
Answers
18 moles of oxygen can be produced by decomposing 12 moles of KClO₃.
How many moles are produced from 12 moles of KClO₃?The balanced chemical equation for the decomposition of KClO₃ is:
2KClO₃ → 2KCl + 3O₂
According to the equation, for every 2 moles of KClO₃ decomposed, 3 moles of O₂ are produced. So, to determine how many moles of O₂ are produced by decomposing 12 moles of KClO₃, we can use the following proportion:
2 moles KClO₃ / 3 moles O₂ = 12 moles KClO₃ / x moles O₂
where x is the number of moles of O₂ produced.
Solving for x, we get:
x = (3 moles O₂)(12 moles KClO₃) / (2 moles KClO₃)
x = 18 moles O₂
Therefore, 12 moles of KClO₃ can produce 18 moles of O₂.
Learn more about moles, here:
https://brainly.com/question/15209553
#SPJ9