Which metal(s) is most likely to accommodate Hatoms (radius 37.0 pm) with the least distortion to the crystalline lattice, given their atomic radii? Choose one or more: titanium (atomic radii - 147 pm) zirconium (atomic radii - 160 pm) hafnium (atomic radii - 159 pm)
Answers
The metal most likely to accommodate H atoms (radius 37.0 pm) with the least distortion to the crystalline lattice is Zirconium (atomic radii - 160 pm).
When comparing the atomic radii of the given metals (Titanium - 147 pm, Zirconium - 160 pm, Hafnium - 159 pm) with the H atom radius (37.0 pm), we should choose the metal with the largest atomic radius. A larger atomic radius indicates more space within the lattice to accommodate H atoms, thus causing less distortion.
Among the given metals, Zirconium has the largest atomic radius, making it the most suitable metal to accommodate H atoms with minimal distortion to the crystalline lattice.
To know more about crystalline lattice, visit:
https://brainly.com/question/7563411
#SPJ11
Related Questions
i will put the questions in the comments that i need help with*
Answers
What is the mass of 2.30 x 10^24 particles of water, H₂O?
Answers
The mass of 2.30 x 10²⁴ particles of water (H₂O) is 60.84 grams.
What exactly is mass defined as?The amount of matter in a particle or object is represented by its mass, which is denoted by the symbol m. In the International System (SI), the kilograms serves as the default unit of mass (kg).
The formula N = n L describes the relationship between a substance's number of particles and number of moles.
n also defined using the equation n = m/M
N = n L
N = m/M L
Then substitute N and L values,
2.30×10²⁴ = m/M × 6.02×10²³
Molar mass of water = 18
2.30×10²⁴ = m/18 × 6.02×10²³
2.30×10²⁴/6.02×10²³ = m/18
3.38 = m/18
m = 3.38 × 18
m = 60.84grams.
To know more about mass visit:
https://brainly.com/question/11959999
#SPJ1
5.) how many milliliters of 0.100 m naoh(aq) would be needed to completely neutralize 50.0 milliliters of 0.300 m hcl(aq)?
Answers
150 mL of 0.100 M NaOH is needed to completely neutralize 50.0 mL of 0.300 M HCl. To determine the amount of NaOH needed to neutralize the HCl, we must first balance the chemical equation. HCl + NaOH → NaCl + H2O. The balanced equation tells us that one mole of NaOH reacts with one mole of HCl.
We can use the formula M1V1 = M2V2 to calculate the amount of NaOH needed. First, we determine the number of moles of HCl present in 50.0 mL of 0.300 M HCl:
0.300 mol/L x 0.0500 L = 0.0150 moles HCl
Since one mole of NaOH is needed to neutralize one mole of HCl, we need 0.0150 moles of NaOH.
Now, we can use the concentration of the NaOH solution to calculate the volume needed:
0.100 mol/L x V = 0.0150 moles
V = 0.0150 moles / 0.100 mol/L = 0.150 L = 150 mL
Therefore, 150 mL of 0.100 M NaOH is needed to completely neutralize 50.0 mL of 0.300 M HCl.
To know about neutralize :
https://brainly.com/question/14156911
#SPJ11
How many liters of water are needed to prepare a 1.67M solution of Ba(OH)2 if you need to dissolve 235g of it?
Answers
Answer: 2.04
Explanation:
Answer:
Approximately \(0.821\; \rm mol\).
Explanation:
Look up the relative atomic mass of \(\rm Ba\), \(\rm O\), and \(\rm H\) on a modern periodic table:
\(\rm Ba\): \(137.327\).\(\rm O\): \(15.999\).\(\rm H\): \(1.008\).Calculate the formula mass of \({\rm Ba(OH)_2}\):
\(\begin{aligned}& M({\rm Ba(OH)_2}) \\ &= 137.327 + 2\times(15.999 + 1.008) \\ &\approx 171.334\; \rm g \cdot mol^{-1}\end{aligned}\).
Calculate the number of moles of \({\rm Ba(OH)_2}\) formula units in that \(235\; \rm g\) of this compound:
\(\begin{aligned}& n({\rm Ba(OH)_2}) \\ &= \frac{m({\rm Ba(OH)_2})}{M({\rm Ba(OH)_2})} \\ &= \frac{235\; \rm g}{171.334\; \rm g \cdot mol^{-1}} \approx 1.37159\; \rm mol \end{aligned}\).
Calculate the volume of a \(c({\rm Ba(OH)_2}) = 1.67\; \rm mol \cdot L^{-1}\) with approximately \(n({\rm Ba(OH)_2}) = 1.37159\; \rm mol\) of the solute:
\(\begin{aligned}& V({\rm Ba(OH)_2}) \\ &= \frac{n({\rm Ba(OH)_2})}{c({\rm Ba(OH)_2})} \\ &= \frac{1.37159\; \rm mol}{1.67\; \rm mol \cdot L^{-1}} \approx 0.821\; \rm L \end{aligned}\).
Convert to scientific notation : 520,000,000
Answers
Answer:
5.2 x 10^8
Explanation:
Answer:
5.2x10^8
Explanation:
hope this helped
I need some help with this. I’m so confused on what to do and an explanation to what the answer is and why would be ideal.
Answers
Answer:
who do I submit my questions to there is no submit or done sign I download this app for help not to waste my money and time. I need help with math please don't let us start off on the wrong foot
why does the equilibrium strongly favor the reverse reaction hydration of the alkene
Answers
The equilibrium for the hydration of alkenes strongly favors the reverse reaction due to thermodynamic factors.
When an alkene is hydrated, an alcohol is formed by adding water across the double bond. This reaction is exothermic and releases energy. As a result, the entropy of the system decreases since fewer molecules are present in the products than in the reactants.
In contrast, the reverse reaction is endothermic and requires energy to proceed. This results in an increase in the entropy of the system since more molecules are present in the products than in the reactants.
Therefore, the equilibrium for the hydration of alkenes strongly favors the reverse reaction due to the thermodynamic factors of energy and entropy.
You can learn more about reverse reaction at
https://brainly.com/question/14611993
#SPJ11
A cube with a mass of 250. 0 g has sides measuring 5. 00 cm each. What is the density of this cube in grams per cubic centimeter?
Answers
The actual formula for volume for a cube is the length multiplied by the width and then multiplied by the height. Since all three measurements are the same, the formula results in the measurement of one side cubed. For the example, 5^3 is 125 cm^3. Multiply the volume by the known density, which is the mass per volume.
How many liters of wine can be held in a wine barrel whose capacity is 30.0 gal? You had been given a new penny to test if it is made up of pure copper or not. You measured the mass of the penny which was 2.49 g. You then find that the penny displaces 0.349 cm3 of water. Is the penny made of pure copper? (Density of pure copper = 8.96 g/cm3)
Answers
The first step in this calculation is to know how many liters is equal to 1 gallon, and the value is 3.785 liters, so now we have to make the following calculation:
1 gal = 3.785 Liters
30.0 gal = x Liters
x = 3.785 * 30.0
x = 114 Liters
Question 5
Calculate the wavelength (in nm) of a neutron traveling at a speed of 85.0 m/s. The mass of a neutron is 1.67493 × 10–24 g.
1 point
4.65 nm
8.58 nm
4.96 × 10–3 nm
5.78 × 10–3 nm
Answers
The wavelength of the neutron is 4.65 n. Wavelength is typically measured in units of meters (m), centimeters (cm), or nanometers (nm).
What is Wavelength?
Wavelength is the distance between successive crests, troughs, or any other equivalent points in a wave. It is usually denoted by the Greek letter lambda (λ) and is commonly used to describe electromagnetic waves, such as light, as well as other types of waves, including sound and water waves.
Since the wavelength of a neutron is given by λ = h/p, where h is Planck's constant and p is the momentum of the neutron, we can calculate the wavelength using the equation λ = h/mv, where m is the mass of the neutron, v is its velocity, and h is Planck's constant.
First, we need to convert the velocity from m/s to cm/s, since the units of h are cm^2 g/s:
85.0 m/s × (100 cm/1 m) = 8500 cm/s
Next, we can plug in the values for m, v, and h:
λ = h/mv = (6.626 × 10^-27 cm^2 g/s)/(1.67493 × 10^-24 g × 8500 cm/s)
λ = 4.65 nm
Therefore, the wavelength of the neutron is 4.65 nm.
Learn more about Wavelength from given link
https://brainly.com/question/10750459
#SPJ1
In what form does the sun's energy arrive on Earth?The physical characteristics of two large predatory animal species are shown in the table below.
Physical Characteristics
Species Characteristics
X Short canine teeth, small prey size
Y 1-foot-long canine teeth, large prey size
Which species will most likely survive if the prey population in the area decreases?
Species X because it is difficult to bring down a small prey
Species X because short canines are less likely to break
Species Y because long canines are less likely to break
Species Y because it is easy to bring down a large prey
Answers
Answer:
radiation B species x because short canines are less likely to break.
The mass of a single gold atom is 3.27X10^-22 grams. How many gold Adams with there be in 57.8 mg of gold.
Answers
Answer:
18 * 10^19 atoms
Explanation:
We must first convert 57.8 mg to grams.
If 1000 mg = 1g
57.8 mg = 57.8/1000 = 57.8 * 10^-3 g
Now;
If 1 gold atom has a mass of 3.27X10^-22 grams
x gold atoms have a mass of 57.8 * 10^-3 g
x = 57.8 * 10^-3 g/3.27X10^-22 g
x = 18 * 10^19 atoms
Do the following elements represent the same
group, period, or neither?
Li, C, F
F, S, P
O, S, Se
[Choose ]
[Choose ]
[Choose ]
Answers
According to the electronic configuration, Li,C, F belong to same period as they have 2 shells .Among F,S, P it is neither same period or group and for O,S,Se they belong to the same group as they have 6 valence electrons.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ1
Raquel would like to describe the observable properties of water. What properties can she use?
A Colorless and rough
B Colorless and smooth
C Smooth and rough
D Soft and smooth
HELP ASAP
Answers
Answer:
b
Explanation:
water is generally transparent and smooth to the touch unless it is frozen, then it is rough
PLEASE HELP DUE IN 15!!!!!!!!!!!! WILL GIVE BRAINLIEST!!
Question 17: 2C2H6 + 7O2 ---> 4CO2 + 6H20
How many moles of O2 are needed to completely react with 4.50 moles of C2H6 according to the above equation?
a. 5.8 moles
b. 3.11 moles
c. 1.29 moles
d. 63.0 moles
Answers
Explanation:
n=,4.5 moles C2H6
the ratio 2:7
4.5:x
x=15.75 moles
When table salt (sodium chloride which ionizes into Na and Cl ) is added to alginate, a geldoes not form and spherification does not occur. This happens because:L.✓Alginate requires a doubly charged cation to crosslinkM. The salt is negatively charged and repels the alginateN.The alginate is a doubly charged anionO.✓The salt only has one positive charge that neutralizes the negative charge in thealginate
Answers
When table salt (sodium chloride which ionizes into Na and Cl) is added to alginate, a gel does not form and spherification does not occur. This happens because the salt only has one positive charge that neutralizes the negative charge in the alginate.
There are various types of Spherification. Spherification is the creation of small spheres with a thin film on the surface and a liquid center. The process of spherification is mostly used in molecular gastronomy to make small, flavorful balls of liquid ingredients that burst in the mouth when bitten into. The method involves a process of encapsulating liquid droplets in a sphere made of a gel-like film. This process requires sodium alginate (E401), a gel-forming ingredient that thickens the liquids.
Sodium alginate gelation occurs as a result of the mixture of an alginate solution with a cation solution that causes the solution to gel. The sodium ions present in the solution swap with calcium ions present in the cation solution, causing a gel to form. This occurs as a result of a chemical reaction known as cross-linking. When table salt is added to the alginate solution, a gel does not form and spherification does not occur since the salt only has one positive charge that neutralizes the negative charge in the alginate. Alginate requires a doubly charged cation to cross-link.
Learn more about cations at https://brainly.com/question/14309645
#SPJ11
0.00170 mol of hydrogen was collected over water. if the total pressure of the gases was 749.0 mmhg and the vapor pressure was 21.5 mmhg
Answers
The moles of water vapor in the mixture are 0.00165 mol.
To find the moles of water vapor in the mixture, we need to consider the total pressure of the gases and the vapor pressure of water.
The total pressure of the gases (P_total) is given as 749.0 mmHg, and the vapor pressure of water (P_water) is given as 21.5 mmHg.
The pressure exerted by the water vapor in the mixture (P_vapor) can be calculated by subtracting the vapor pressure from the total pressure:
P_vapor = P_total - P_water
= 749.0 mmHg - 21.5 mmHg
= 727.5 mmHg
Now, we can use the ideal gas law to calculate the moles of water vapor (n_vapor). The ideal gas law equation is:
PV = nRT
Where:
P is the pressure (in atm or mmHg),
V is the volume (in liters),
n is the number of moles,
R is the ideal gas constant (0.0821 L·atm/(mol·K)),
T is the temperature (in Kelvin).
Since we are given the pressure (P_vapor), volume is not specified, and temperature is assumed to be constant, we can simplify the equation to:
n_vapor = P_vapor / (RT)
To use this equation, we need to convert the pressure from mmHg to atm and the temperature to Kelvin. Assuming the temperature is known and constant, let's use 298 K.
Converting pressure to atm:
P_vapor = 727.5 mmHg * (1 atm / 760 mmHg)
= 0.957 atm
Now we can calculate the moles of water vapor:
n_vapor = 0.957 atm / (0.0821 L·atm/(mol·K) * 298 K)
≈ 0.00165 mol
Therefore, the moles of water vapor in the mixture are approximately 0.00165 mol.
The moles of water vapor in the mixture are approximately 0.00165 mol.
To know more about moles, visit;
https://brainly.com/question/29367909
#SPJ11
0.00170mol of H_(2) was collected over water. If the total pressure of the gases was 749.0mmHg and the vapor pressure was 21.5mmHg, find the moles of water vapor in the mixture.
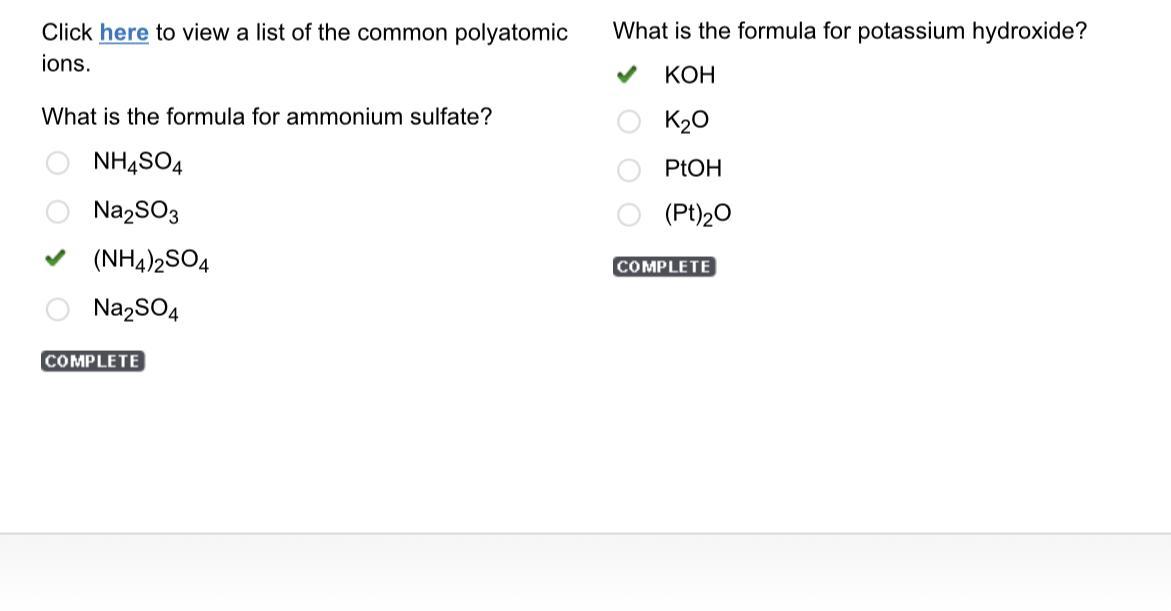
Click here to view a list of the common polyatomic ions. what is the formula for ammonium sulfate? nh4so4 na2so3 (nh4)2so4 na2so4
Answers
Option C ;(NH4)2SO4 is the formula for ammonium sulfate.
It is an inorganic salt. It appears as fine white crystals. Its molecular weight is 132.14g/mol. Its density is 1.77g/cm³.It has many commercial uses. It is mainly used as fertilizer for alkaline soils because ammonium ions forms small amounts of acid which lowers the pH of the soil.
It is acidic with pH of 5.5. Its melting point is 235 °C. it is soluble in water. It is obtained by the reaction of sulfuric acid with ammonia. Sulfuric acid will react with two equivalents of ammonia.
To know more about ammonium sulfate click here
brainly.com/question/2671989
#SPJ4

Answer:
C. (NH4)2SO4
And the second question is
A. KOH
Explanation:
The volume of a gas must be measured at several points during an experiment. Which units should be used to describe the volume of the gas?
liters
centimeters
degrees Celsius
milligrams
Answers
Answer:liters
Explanation:
Please submit this as soon as possible!
Answers
Answer: 10=14 1=t6
Explanation:
How many electrons are represented by a dash (-) in a lewis dot structure compound?
A. 2
B. 4
C.3
D. 1
Answers
The dash represents two electrons
The nuclear mass of 141Ba is 140.883 amu. Calculate the binding energy per nucleon for 141Ba. In this problem, to avoid rounding errors, use the constants Sapling gives you in the hint below, not the constants used in my notes. Also, be sure to use all decimal values provided
Answers
The binding energy per nucleon for 141Ba is approximately 1.350 x 10^-13 J/nucleon.
To calculate the binding energy per nucleon for 141Ba, we'll follow the steps outlined earlier:
Given:
Nuclear mass of 141Ba = 140.883 amu
Using the atomic masses from the periodic table:
Mass of proton = 1.007276 amu
Mass of neutron = 1.008665 amu
Number of protons (Z) = 56 (atomic number of barium)
Number of neutrons (N) = (140.883 - 56) = 84
Step 1: Calculate the mass defect (Δm):
Sum of proton masses = 56 x 1.007276 amu
Sum of neutron masses = 84 x 1.008665 amu
Δm = (Sum of proton masses + Sum of neutron masses) - Actual nuclear mass
Step 2: Calculate the binding energy (BE):
Binding Energy (BE) = Δm x c^2
where c = 2.998 × 10^8 m/s
Step 3: Calculate the binding energy per nucleon:
Binding Energy per Nucleon = BE / (Z + N)
Performing the calculations:
Sum of proton masses = 56 x 1.007276 amu = 56.446656 amu
Sum of neutron masses = 84 x 1.008665 amu = 84.68586 amu
Δm = (56.446656 amu + 84.68586 amu) - 140.883 amu = 0.249516 amu
BE = (0.249516 amu) x (2.998 × 10^8 m/s)^2 = 2.23899 x 10^-11 J
Binding Energy per Nucleon = (2.23899 x 10^-11 J) / (56 + 84) = 1.350 x 10^-13 J/nucleon.
To know more about proton visit:
brainly.com/question/2449552
#SPJ11
16. Find the molarity (M) of a solution prepared
by diluting 67.5 mL of 6.0 M NaOH to 450 mL.
Answers
Answer:
M = (6.0 M x 67.5 mL) / 450 mL
M = 0.9 M
Does this action make a physical or chemical change: hydrogen burns in chlorine gas
Answers
Answer:
I CANT
Explanation:
Answer:
phisical i think
Explanation:
chemical would be baking powder in a pancake, the pancake would be in turn more brown cause of baking powder mixing with flower.
what does Le châteliers principle state?
Answers
Hope this helps!
Two samples A and B of chloride of gold contains 15.1% and 35.1% chloride respectively show that these figures are in agreement with the law of multiple proportion (Au=197, cl=35.5)
Answers
Considering the law of multiple proportions, they obey the law of multiple proportion.
The law of multiple proportions or Dalton's law establishes that if two elements form more than one compound, establishing the composition of one of them, the other element will be in the ratio of natural numbers (integers and simple).
In other words, when two elements form more than one compound, the masses of one of them that combine with the same mass of the other element are in a simple relationship, expressible by a quotient of whole numbers.
In this case, two samples A and B of chloride of gold contains 15.1% and 35.1% chloride respectively.
This is, A contains 15.1% of Cl and 84.9% of Au . So:
\(\frac{84.9}{15.1}= 5.6\)
This indicates that there is 5.6 g of Au per gram of Cl.
And B contains 35.1% of Cl and 64.9% of Au . So:
\(\frac{64.9}{35.1}= 1.8\)
This indicates that there is 1.8 g of Au per gram of Cl.
Then, the relationship can be expressed as:
\(\frac{5.6}{1.8}=\frac{3}{1}\)
Since the composition of the atoms in the compounds are in simple ratio to each other, they obey the law of multiple proportion.
Learn more about law of multiple proportion:
https://brainly.com/question/26090646
https://brainly.com/question/25260214
- A compound contains 4.56g. of Pb, 2.3g Cl, and 3.1g 0. What is the percent
composition for each element?
please show work
Answers
4.56 of Pb divided by 9.96 = 0.4578313 now multiply by 100 and get 45.8% of Pb, then do the same for the rest of the elements, hope this helped <3
(MATLAB)
An experiment was conducted to see if chemicals sprayed on clouds for artificial rainfall could reduce the occurrence of hail.
Among the cloudy days in the area where the hail was found, 50 days were observed with chemicals sprayed on the clouds, and 165 days were observed with nothing on the clouds.
Number of days the drug was administered Number of days without medication
hail day 7 43
a day without hail 43 122
Find a 90% confidence interval for the difference in the incidence of hail between spraying and not spraying the cloud. If tea is not sprinkled - count as spraying medicine, enter a smaller value in the confidence interval in the answer entry window
Answers
The 90% confidence interval for the difference in the incidence of hail between spraying and not spraying the clouds is (-0.141, 0.241).
Does the experiment show a significant difference in hail occurrence between spraying and not spraying the clouds?The experiment aimed to determine if spraying chemicals on clouds for artificial rainfall could reduce hail occurrence. The data collected included the number of days when chemicals were sprayed on the clouds and the number of days when no intervention was done.
Out of the observed cloudy days with hail, 7 days had spraying while 43 days had no spraying. On the other hand, out of the observed cloudy days without hail, 43 days had spraying and 122 days had no spraying.
To calculate the 90% confidence interval for the difference in hail incidence, we use a formula that takes into account the sample sizes and proportions. After performing the calculations, the resulting confidence interval is (-0.141, 0.241). This means that there is not enough evidence to conclude a significant difference in hail occurrence between spraying and not spraying the clouds, as the confidence interval contains zero.
Learn more about hail
brainly.com/question/31816448
#SPJ11
A student dissolves 20g of potassium chloride in 100cm³ of water in a beaker. What is the concentration of the solution in g/dm³?
Answers
Answer:
The concentration of the solution is 2 g/dm³
Explanation:
The equation used to find the concentration of a solution is "c = m/v"
C= concentration
M= mass
V= volume
The question asks for the answer to be in decimeters, so you need to 100 cm³ to dm³. To do this, you divide 100 by 10, giving you 10 cm³. Then you just plug in your numbers into the formula: c = 20g/10dm³.
The concentration of the potassium chloride solution has been \(\rm 2\;\times\;10^-^3\;g/dm^3\).
The concentration has been defined as the mass of sample present in a definite volume of the solution. The concentration has been given by the moles, molarity, g/ml, g/L etc.
The concentration attained by the student has been by dissolving 20 g of KCl in \(\rm 100\;cm^3\) water has been given in \(\rm g/cm^3\) as:
\(\rm \dfrac{g}{cm^3}=\dfrac{20\;g}{100\;cm^3}\\ \dfrac{g}{cm^3}=0.2\;g/cm^3\)
The concentration of the solution obtained by the student has been \(\rm 0.2\;g/cm^3\).
The conversion of \(\rm cm^3\;to\;dm^3\) has been given as:
\(\rm 1\;g/cm^3=0.001\;g/dm^3\)
The concentration of \(\rm 0.2\;g/cm^3\) solution has been given as:
\(\rm 1\;g/cm^3=0.001\;g/dm^3\\0.2\;g/cm^3=0.2\;\times\;0.001\;g/dm^3\\0.2\;g/cm^3=2\;\times\;10^-^3\;g/dm^3\)
The concentration of the potassium chloride solution has been \(\rm 2\;\times\;10^-^3\;g/dm^3\).
For more information about concentration of solution, refer to the link:
https://brainly.com/question/202460
he long run equilibrium condition for perfect competition is:
a. P=AVC=MR=MC.
b. Q=AVC=MR=MC.
c. Q=ATC=MR=MC.
d. P=ATC=MR=MC.
Answers
Option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
The long-run equilibrium condition for perfect competition is that price (P) is equal to average total cost (ATC), which is also equal to marginal cost (MC), and marginal revenue (MR).
Option (d), P=ATC=MR=MC, best represents the long-run equilibrium condition for perfect competition. In perfect competition, firms operate at the minimum point of their average total cost curve, where price equals both average total cost and marginal cost. This condition ensures that firms are earning zero economic profit and are producing at an efficient level.
In the long run, if firms are earning economic profit, new firms will enter the market, increasing competition and driving prices down. Conversely, if firms are experiencing losses, some firms may exit the market, reducing competition and causing prices to rise. This process continues until firms reach a state where price equals average total cost, marginal cost, and marginal revenue, ensuring a long-run equilibrium.
Therefore, option (d), P=ATC=MR=MC, accurately represents the long-run equilibrium condition for perfect competition, reflecting the balance between price and cost for firms operating in a competitive market.
Know more about Equilibrium here:
https://brainly.com/question/30694482
#SPJ11