Which is true regarding percentage error?
The percentage error is calculated by multiplying the approximation value and the exact value.
The percentage error is a percentage that details how far an approximation is from the exact value after an experiment.
The percentage error is a set of inferences made by human senses and scientific equipment.
The percentage error is a percentage that states how many mistakes were made during an experiment.
Answers
The statement that is true regarding percentage error is as follows: the percentage error is a percentage that details how far an approximation is from the exact value after an experiment (option B).
What is percentage error?Percentage error is the difference between estimated value and the actual value in comparison to the actual value and is expressed as a percentage.
The percentage error in an experiment can be calculated by subtracting the actual value from the estimated value divided by the actual value, then multiplying the result by 100.
Percentage error = (Estimated value - Actual value/ Actual value) × 10
Therefore, the statement that is true regarding percentage error is as follows: the percentage error is a percentage that details how far an approximation is from the exact value after an experiment.
Learn more about percentage error at: https://brainly.com/question/4170313
#SPJ1
Related Questions
A student uses a magnet to move a 0.025 kg metal ball. The magnet exerts a force of 5 N, which causes the ball to begin moving. What is the acceleration of the ball when it begins to move?
Type your answer...
Answers
Answer: The acceleration of the ball is 200 m/s².
Explanation:
Force:
Force is the push or pull on an object with mass that causes it to change velocity or to accelerate.
Force represents as a vector, which means it has both magnitude and direction.
Given:
Mass of metal ball = 0.025 kg
Force = 5 N
To Find:
Acceleration, = ?
Solution:
Formula:

draw the major thermodynamic and kinetic products of the reaction. draw the kinetic product. select draw rings more erase select draw rings more erase select draw rings more erase c h br draw the thermodynamic product.
Answers
The major thermodynamic product of the reaction between C₅H₈ and HBr is the most stable product, which is usually the one with the lowest free energy. On the other hand, the major kinetic product is the product that is formed through a faster and less controlled reaction pathway.
This thermodynamic product product is formed through a slower and more controlled process, which allows for the formation of stronger chemical bonds and results in a more stable final product.
This kinetic product is formed due to its lower activation energy, which makes it easier to form, but it may not necessarily be the most stable product.
In the case of C₅H₈ and HBr, the reaction can result in both thermodynamically and kinetically stable products, meaning that there can be multiple products formed, each with different stability properties. The thermodynamically stable product is the one with the lowest free energy, while the kinetically stable product is the one with the lower activation energy.
In summary, the major thermodynamic product of the reaction is the most stable product, while the major kinetic product is the product that is formed through a faster and less controlled reaction pathway. However, it is important to note that the exact products of the reaction can vary based on the reaction conditions and reactant concentrations.
To know more about kinetic products click on below link:
https://brainly.com/question/22616533#
#SPJ11
Complete question:
What are themajor thermodynamic and kinetic products of the reaction.
C₅H₈ + HBr (1 equivalent) ------> thermodynamically stable + Kinetically stable
The German
(1868-1934;
Prize in
Chemistry
1918) was
able to
synthesize ammonia (NH3) by
reacting 0.1248 M H₂ and 0.0416 M
N₂ at about 500°C. At equilibrium, the
mixture contained 0.00272 M NH3.
What is K for the reaction N₂ + 3H₂ =
2NH3at this temperature? What is Kp?
chemist Fritz Haber
Nobel
Answers
At 298 K, the reaction N2(g) + 3H2(g) 2NH3(g) results in an enthalpy change (H) of -92.38 kJ.
What is the effect of temperature on the equilibrium N2 3H2 ⇋ 2NH3?N2(g) + 3H2(g) 2NH3(g) + Heat is the equation for our equilibrium reaction. Think about increasing the nitrogen gas concentration. Since the reaction is pushed to the right when nitrogen gas concentration is increased, we may be certain that the equilibrium will shift to the right.Kp for the N2 + 3H2 2NH3 reaction at 400°C is 1.64 10-4. Iron catalyst addition will not change the reaction's state of equilibrium. The general statement: Kp = Kc(RT) (RT) It is possible to derive n by taking into account the moles of gaseous products and reactants.N2 (g) + 3 H2 (g) 2 NH3 (g) If equal amounts of each reactant are utilised, hydrogen will be the limiting agent because it takes 3 moles of hydrogen to produce 1 mole of ammonia.To learn more about enthalpy refer to:
https://brainly.com/question/18849238
#SPJ1
A sample of hydrogen gas occupies a volume of 300 ml at 1.2 bar pressure and 25 degree C. Calculate its volume at 0.45 bar pressure and 700 C
Answers
Answer
2612 mL
Explanation
Given:
The initial volume, V₁ = 300 mL
The initial pressure, P₁ = 1.2 bar
The initial temperature, T₁ = 25⁰C = (25⁰C + 273) = 298 K
The final pressure, P₂ = 0.45 bar
The final temperature, T₂ = 700⁰C = (700⁰C + 273) = 973 K
What to find:
The final volume, V₂ of the gas at 0.45 bar pressure and 700⁰C.
Step-by-step solution:
The question is a volume, pressure, and temperature relationship.
The final volume, V₂ of the gas at 0.45 bar pressure and 700⁰C can be calculated using the Combine gas law equation.
\(\begin{gathered} \frac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} \\ \\ V_2=\frac{P_1V_1T_2}{T_1P_2} \end{gathered}\)Substituting the values of the parameters into the formula
\(\begin{gathered} V_2=\frac{1.2\text{ }bar\times300\text{ }mL\times973\text{ }K}{298\text{ }K\times0.45\text{ }bar} \\ \\ V_2=\frac{350280\text{ }mL}{134.1} \\ \\ V_2=2612\text{ }mL \end{gathered}\)Hence, the volume of the hydrogen gas sample at 0.45 bar pressure and 700⁰C is 2612 mL.
Calculate the concentration of NO2NO2 in an equilibrium mixture given that the concentration of N2O4=0.200MN2O4=0.200M and Keq=67.3Keq=67.3 for the reaction: 2NO2(g)⇌N2O4(g)2NO2(g)⇌N2O4(g). Calculate the concentration of in an equilibrium mixture given that the concentration of and for the reaction: . 18.3 M 0.00297 M 337 M 0.0545 M none of the above
Answers
Answer: The concentration of \(NO_{2}\) in the given equilibrium mixture is 0.0545 M.
Explanation:
The ratio of concentration of products and reactants raised to the power of their coefficients is called equilibrium constant. The symbol used to denote equilibrium constant is \(K_{eq}\).
As the given reaction equation is as follows.
\(2NO_{2}(g) \rightleftharpoons N_{2}O_{4}(g)\)
The expression for equilibrium constant of this reaction is as follows.
\(K_{eq} = \frac{[N_{2}O_{4}]}{[NO_{2}]^{2}}\)
Now, substitute the given values into above formula as follows.
\(K_{eq} = \frac{[N_{2}O_{4}]}{[NO_{2}]^{2}}\\67.3 = \frac{(0.2)}{[NO_{2}]^{2}}\\So, [NO] = \sqrt{\frac{0.2}{67.3}}\\= 0.0545 M\)
Thus, we can conclude that the concentration of \(NO_{2}\) in the given equilibrium mixture is 0.0545 M.
What is epoxy?
....................
Answers
Answer:
1. any class of adhesives, plastics, or other materials that are polymers of epoxies.
2. something like glue or resin
Explanation:
that's the dictionary definition of epoxy.
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
Which statements are true about catalysts

Answers
The true statements about catalysts are the statement 1,2 and 3.
1. Catalysts increase the rate of reaction: Catalysts facilitate chemical reactions by providing an alternative reaction pathway with lower activation energy. They enhance the rate of the reaction without being consumed in the process.
2. Catalysts behave as reactants in the reaction mixture: Catalysts participate in the reaction by interacting with the reactants. They form temporary bonds with the reactant molecules, leading to the formation of an intermediate complex that ultimately results in the desired products.
3. Catalysts decrease the activation energy of a reaction: Catalysts lower the energy barrier required for a reaction to occur by providing an alternative pathway with a lower activation energy. This enables the reactants to overcome the energy barrier more easily, thus increasing the reaction rate.
4. Catalysts show no physical change at the end of the reaction: Catalysts are not consumed or permanently altered in the reaction. They remain chemically unchanged and are available to participate in subsequent reaction cycles.
The statement "Catalysts are required in large concentrations in a reaction" is false. Catalysts work effectively even in small concentrations, as their role is to facilitate the reaction rather than being directly involved in the stoichiometry of the reaction.
For more questions on catalysts, click on:
https://brainly.com/question/12507566
#SPJ8
Paraffin softens on a hot plate.
Is it crystalline or amorphous?
Answers
14. Which group below correctly list bases that completely dissociate in water?
O a. NaOH, KOH, LIOH
O b. NH3, NH4OH, HCI
C. NaOH, H3PO4, MgOH
O d. NaOH, KOH, H2CO3
15. When the oxidation number increases the element is
decreases the element is
and if the oxidation number
O a. reduced, reduced
O b. reduced, oxidized
O c. oxidized, oxidized
O d. oxidized, reduced
16. The oxidation number for O in NaHCO3 is
O a. 1-
O b. 1+
O c. 2-
O d. 2+
17. The oxidation number for Na, Cr, and o respectively in the compound Na2Cro4
O a. 6+, 1+, 2+
O b. 1+, 6+, 2-
O c. 2-, 1+, 6+
O d. 1-, 2-, 3+
18. CuSO4 + 2NaOH --> Cu(OH)2 + Na2S04
The reaction above represents
O a. Oxidation
O b. Reduction
O c. Neutralization
O d. None of the above
19. A balloon with a volume of 5.0 L is filled with a gas at 2 atmospheres. If the pressure is reduced
to 1 atmospheres without a change in temperature, what would be the volume of the balloon?
O a. 5.0 L
b. 10 L
O c. 12 L
O d. 10 ml
Answers
Answer:14=D
15=B
16=D
17=C
18=A
19=D
Explanation: I did this and got a 100% °³°
Does anybody know these questions ?

Answers
Answer:
proton 5
Explanation:
I think it is fluorine
Below is a 3D representation of a cyclohexane (C6H12) molecule! a cyclic compound used in the manufacture of nylon and found in the distillation of petroleum. What is the molecular geometry around each carbon atom? Be sure to rotate the molecule to see all atoms.
Answers
The geometry around the carbon for sp3 hybridized atom is tetrahedral. The geometry around the carbon for sp2 hybridized atom is planar triangular. The geometry around the carbon for sp hybridized atom is linear.
Before overlapping with 1s orbital of hydrogen, first the atomic orbitals of carbon undergoes hybridization to form hybrid orbitals. The hybrid orbitals involve in bond formation with hydrogen. Hence, the geometry around each carbon atom in the molecule depends on the type of hybridization it undergoes.
The geometry around the carbon for sp3 hybridized atom is tetrahedral. The geometry around the carbon for sp2 hybridized atom is planar triangular. The geometry around the carbon for sp hybridized atom is linear.
To learn more about hybridization check the link below:
https://brainly.com/question/22765530
#SPJ4
Complete question:
Below is a 3D representation of a cyclohexane (C6H12) molecule! a cyclic compound used in the manufacture of nylon and found in the distillation of petroleum. What is the molecular geometry around each carbon atom? Be sure to rotate the molecule to see all atoms.

What is heat transferred from movement of fluids in a circular motion called
Answers
Answer: it's known as heat transfer
Explanation: Heat always moves from a warmer substance to a cooler substance. For example, holding an ice cube will make your hand begin to feel cold in a few seconds. But is the coldness in the ice cube moving to your hand? No! Since cold is the absence of heat, it’s the heat in your hand that moves to the ice cube. This is one of the ways that heat is transferred.
According to the concept of thermal energy, heat transfer in a circular motion is called as convection.
What is thermal energy?
Thermal energy is defined as a type of energy which is contained within a system which is responsible for temperature rise.Heat is a type of thermal energy.It is concerned with the first law of thermodynamics.
Thermal energy arises from friction and drag.It includes the internal energy or enthalpy of a body of matter and radiation.It is related to internal energy and heat .It arises when a substance whose molecules or atoms are vibrating faster.
These vibrating molecules and atoms collide and as a result of which heat is generated in a substance , more the collision of particles , higher is the thermal energy.There are three types of thermal energy.
Learn more about thermal energy,here:
https://brainly.com/question/3022807
#SPJ6
When a chemical reaction occurs blank happens
Answers
Answer:
In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
What are 3 elements similar to silver
Answers
Answer:
Copper, Sodium, and Francium
Explanation:
Which of the following is a possible
way to describe the SO3 component in
the reaction below?
Sa(s) + 120₂(g) → 8SO3(g)
A. 8 atoms SO3
B. 8 molecules SO3
C. 80.07g SO3
D. 32 LSO3
Answers
The correct answer is B. 8 molecules \(SO_3\). Option B
In the given reaction:
S(s) + \(O_2\)(g) → \(SO_3\)(g)
The stoichiometric coefficient in front of the \(SO_3\)molecule is 8, which indicates that 8 molecules of \(SO_3\)are formed as a product. This coefficient represents the ratio of the number of molecules involved in the reaction.
Option A (8 atoms \(SO_3\)) is incorrect because it only mentions the number of atoms, not molecules. The stoichiometric coefficient does not represent the number of atoms, but rather the number of molecules.
Option C (80.07g \(SO_3\)) is incorrect because it mentions a specific mass. The stoichiometric coefficient does not directly represent the mass of the substance, but rather the relative amount of molecules involved in the reaction.
Option D (32 \(SO_3\)) is incorrect because it mentions a specific volume. The stoichiometric coefficient does not directly represent the volume of the substance, but rather the relative amount of molecules involved in the reaction.
Therefore, the correct way to describe the \(SO_3\)component in the reaction is option B: 8 molecules \(SO_3\). This represents the ratio of the number of molecules of \(SO_3\)that are produced in the reaction.
Option B
For more wsuch question on molecules visit:
https://brainly.com/question/475709
#SPJ8
A student made a sketch of a potential energy diagram to represent a reaction with a -^H
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain
what the values of X and Y represent.

Answers
Answer: It’s correct because it’s showing an exothermic reaction. x is the reactants and y is the products.
Explanation: -ΔH means the reaction is exothermic and releasing heat. This lowers the potential energy.
Answer:
answer above is correct
Explanation:
Does fish changes its density compared to humans?
Answers
Answer:
A fish can change its density by expanding or contracting air sacs in their bodies. All fish have densities near the density of water, but just like people have different weights and volumes, so do different fish
Explanation:
HOPE THIS HELPS!
Density is related to ____.
Height
Mass
Length
Shape
Answers
Answer:I believe it’s mass
Explanation:
Which best describes the activation energy on the graph below?

Answers
ANSWER
The vertical difference between line 1 and 2
EXPLANATION;
According to Arrheniu's theory, before the reactants can be converted to products, the reacting particles must possess an energy greater than the activation energy.
Hence, the vertical difference between line 1 and 2 best describe the activation energy.
Therefore, the correct answer is option B
I want the solve of this problem

Answers
Answer:
the answer of this question is B
Solid silver chloride and an aqueous solution of nitric acid are produced when a solution if silver nitrate is reacted with a solution of hydrochloric acid.
Write a word and skeleton equation
Answers
The word and skeleton equations for the reaction involving silver nitrate and hydrochloric acid are:
Silver nitrate + Hydrochloric acid → Silver chloride + Nitric acidAgNO3(aq) + HCl(aq) → AgCl(s) + HNO3(aq)Equation of reactionsA word equation represents a chemical reaction using the names of the substances involved in the reaction.
On the other hand, a skeleton equation is a more concise representation of a chemical reaction, using chemical formulas and symbols instead of the names of the substances involved in the reaction.
Thus, for the reaction involving silver nitrate and hydrochloric acid
Word equation: Silver nitrate + Hydrochloric acid → Silver chloride + Nitric acidSkeleton equation: AgNO3(aq) + HCl(aq) → AgCl(s) + HNO3(aq)
More on equation of reactions can be found here: https://brainly.com/question/16921139
#SPJ1
Nuclear binding energies for the fusion of a mole of nuclei typically correspond to mass differences on the order of:
A. grams
B. milligrams
C. micrograms
D. nanograms
Answers
Answer:
nanograms
Explanation:
Nuclear binding energy refers to the energy needed in order to break up the nucleus of an atom into its component parts: protons and neutrons, it is also the energy used up in the formation of the atom.
The difference between the calculated and actual mass of a nucleus gives its mass defect which is used to calculate the binding energy according to Einstein's equation. The mass differences are usually very small, as little as the order of nanograms.
Where does the oil in cars come from?
Select 2 that apply
-Phytoplankton convert the sugar created through photosynthesis into oil.
-Coral Reefs clean the water by removing any carbon waste, they then convert this into oil.
-Every time there is an earthquake, oil is being produced and new oil deposits are created.
-The phytoplankton that is dead become liquefied over time due to pressure, and oil deposits
are formed.
-Fish produce oil as a waste product that eventually forms oil deposits.
Answers
- The phytoplankton that is dead become liquefied over time due to pressure, and oil deposits are formed.
- Fish produce oil as a waste product that eventually forms oil deposits.
- If x moles of electrons are transferred when 4.8 g of oxygen reacts with 4.8 g of
magnesium, what is the value of x?
Answers
Answer:
sdfffffffff
Explanation:
dfffffgsdsdsdsdsdsdsdsdsdsdsdsdsdsdsd
A mixture of 0.197 mol carbon dioxide and 0.00278 mol water vapor is held at 30.0 C and 2.50 atm. What is the partial pressure of each gas?
Answers

The partial pressure of each gas is the product of its mole fraction and total pressure. The partial pressure of carbon dioxide is 2.46 atm and the partial pressure of water vapor is 0.034.
What is Dalton's law of partial pressures ?Dalton's law of partial pressure states that the partial pressure of the component gases in a mixture is the product of its mole fraction and the total pressure.
Given that, number of moles of carbon dioxide = 0.197 moles.
number of moles of water vapor =0.00278 mole.
mole fraction of carbon dioxide = 0.197 / (0.197 + 0.00278) = 0.98
Total pressure = 2.50 atm
then partial pressure of carbon dioxide = 0.98 × 2.50 = 2.46 atm
Mole fraction of water vapor = 0.00278 / (0.197 + 0.00278) = 0.0139
partial pressure of water vapor = 0.0139 × 2.50 = 0.034 atm.
Therefore, the partial pressure of carbon dioxide and water vapor are 2.46 and 0.034 atm respectively.
To find more on partial pressure, refer here:
https://brainly.com/question/13199169
#SPJ2
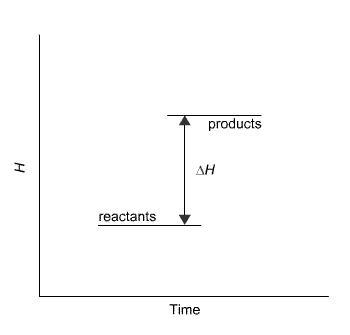
The reaction in the diagram takes place in an ice calorimeter at 0°C.
What happens to the ice?
The temperature of the ice stays the same.
Some ice melts.
The ice gets colder.
You cannot tell from the information given.
Answers
Using the balanced equation CaC₂(ş) + 2 H₂O(1) --> C₂H₂(g) + Ca(OH)₂(aq) how many moles of Ca(OH)2 would be produced if 3.5 moles of H₂O are consumed?
Answers
Answer:
1.75 moles
Explanation:
According to CaC₂(s) + 2 H₂O(l) --> C₂H₂(g) + Ca(OH)₂(aq)
2 moles of H20 will produce 1 mole of Ca(OH)2
therefore 3.5 moles of H2O will produce 3.5 x (1/2) = 1.75 moles of Ca(OH)2
sing Chemical Formulas to Find Number of A
Consider the chemical formula for calcium chlorate: Ca(CIO3)2
How many of each of these atoms is in a molecule of calcium chlorate?
Ca:
CI:
O:
Answers
One calcium atom, two chlorine atoms, and six oxygen atoms make up calcium chlorate.
How many of each of these atoms make up a calcium chlorate molecule?
Calcium chlorate has the chemical formula Ca(ClO3)2. The last number here implies that there are two of everything inside the brackets. A single calcium atom, two chlorine atoms, and six oxygen atoms make up calcium chlorate.
What effects does calcium chloride have on health?Wheezing, tightness in the chest or throat, rash, hives, itching, red, swollen, blistering, or peeling skin with or without fever, difficulty breathing, swallowingfainting or dizzinessmood swingsalteration in the volume of urine excreted.a heartbeat that is out of the ordinary.Learn more about Calcium chlorate here:-
https://brainly.com/question/18425930
#SPJ9
I need help figuring it out the answers were wrong I put in
