Answers
Answer:
Below
Explanation:
Got it right
Check the periodic table, then click electrons

Related Questions
Choose all the answers that apply.
Science
is objective
is subjective
. is based on bias
. is based on fact
. must be ethical
Answers
Answer:
Science is:
objective
based on fact
must be ethical
Explanation:
Science is considered to be objective because it is based on empirical evidence and systematic observation, and it strives to eliminate personal bias and opinions from the scientific process. Science is based on fact, as it relies on repeatable and verifiable experiments to test hypotheses and theories. And, it is important for science to be ethical because the scientific community has a responsibility to ensure that research is conducted in an ethical and responsible manner, and that the results are reported accurately and honestly.
Suppose reactant A reacts with reactant B to form product C according to the balanced equation A + B ⟶ C . If you react 3 moles of reactant A with 1 moles of reactant B, what will be present after the reaction
Answers
If you react 3 moles of reactant A with 1 moles of reactant B, 2 moles of reactant A will be remaining.
From the question given above, the balanced equation for the reaction between reactant A and B is given below:
A + B —> C1 : 1
3 : 1
From the balanced equation above,
1 mole of A reacted with 1 mole of B.
Therefore,
3 moles of A will also react with 3 moles of B.
Thus, B is the limiting reactant and A is the excess reactant.
Finally, we shall determine amount of A remaining.
Amount of A given = 3 moles
Amount of A that reacted = 1 mole
Amount of A remaining =?Amount of A remaining = (Amount of A given) – (Amount of A that reacted)
Amount of A remaining = 3 – 1
Amount of A remaining = 2 molesTherefore, 2 moles of reactant A will remain if 3 moles of reactant A with 1 moles of reactant B.
Learn more: https://brainly.com/question/13476603
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
-300g de acido comercial se disuelve en agua destilada contenidos en un cono, cuyo radio es de 0.005Km y 300cm de altura, si la densidades de 1.2g/m3 ¿Cuál es la concentración expresada en %m/m?
Answers
Answer:
\(\%m/m=76.1\%\)
Explanation:
¡Hola!
En este caso, considerando la información dada, entendemos que se haría primero necesario calcular el volumen del cono en metros cúbicos, teniendo en cuenta que 0.005 km son 5 m y 300 cm son 3 m:
\(V=\frac{1}{3} \pi r^2h\\\\V=\frac{1}{3} \pi *(5m)^2(3m)=78.5m^3\\\\\)
Ahora, convertimos esta cantidad a gramos por medio de la densidad para conocer la masa de la solución:
\(m_{sol}=78.5m^3*\frac{1.2g}{1m^3} =94.2g\)
Finalmente, aplicamos la definición de %m/m para obtener:
\(\%m/m=\frac{300g}{300g+94.2g}*100\%\\\\ \%m/m=76.1\%\)
¡Saludos!
A student made a sketch of a potential energy diagram to represent an endothermic reaction.
A curve line graph is shown. The y axis of the graph has the title Potential Energy and kJ written in parenthesis. The x axis of the graph has the title Reaction Pathway. The curve begins at a higher level and ends at a slightly lower level. A broken horizontal line is shown from a point labelled X on the y axis to the point where the curve begins. Another broken horizontal line is shown from a point labeled Y on the y axis to the point where the curve ends.
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent.
Answers
Based on the description of the potential energy diagram provided, the diagram made by the student appears to be correct.
The potential energy diagram represents the energy changes that occur during a chemical reaction. In an endothermic reaction, the products have higher potential energy than the reactants, meaning energy is absorbed from the surroundings.
The curve line on the graph indicates the energy changes throughout the reaction pathway. It starts at a higher level, representing the initial potential energy of the reactants. As the reaction progresses, the potential energy decreases, indicating the formation of products with lower potential energy.
The broken horizontal line from point X on the y-axis to the point where the curve begins represents the activation energy (Ea) of the reaction. Activation energy is the energy barrier that must be overcome for the reactants to convert into products.
Point X on the y-axis indicates the potential energy of the reactants at the start of the reaction, and the broken line shows the energy required to initiate the reaction.
The broken horizontal line from point Y on the y-axis to the point where the curve ends represents the potential energy of the products. Point Y represents the potential energy of the products at the end of the reaction.
Overall, the student's diagram correctly represents an endothermic reaction, showing the potential energy changes, the activation energy, and the final potential energy of the products. The curve line starts at a higher level (representing the higher potential energy of the reactants) and ends at a slightly lower level (representing the lower potential energy of the products).
For more such questions on potential energy diagram visit;
https://brainly.com/question/23343697
#SPJ8
Determine the molecular formula of a compound that contains 26.7% P 12.1% N and 61.2% Cl and has a molar mass of 812 g/mol
Answers
Considering the definition of empirical and molecular formula, the molecular formula is P₇N₇O₁₄.
Empirical formulaThe empirical formula is the simplest expression to represent a chemical compound, which indicates the elements that are present and the minimum proportion in whole numbers that exist between its atoms, that is, the subscripts of chemical formulas are reduced to the most integers. small as possible.
Molecular formulaThe molecular formula is the chemical formula that indicates the number and type of different atoms present in the molecule. The molecular formula is the actual number of atoms that make up a molecule.
In other words, the molecular formula is the actual formula of the molecule and is made up of the symbols that represent the chemical elements and the subscripts that indicate the number of atoms of each element that participate in the formation of the molecule.
Molecular formula in this caseIn this case, you know:
P: 26.7 %N: 12.1 %Cl: 61.2 %Assuming a 100 grams sample, the percentages match the grams in the sample. So you have 26.7 grams of P, 12.1 grams of N and 61.2 grams of Cl.
Then it is possible to calculate the number of moles of each atom in the molecule, taking into account the corresponding molar mass:
P: \(\frac{26.7 g}{31\frac{g}{mol} }\)= 0.86 moles
N: \(\frac{12.1 g}{14\frac{g}{mol} }\)= 0.86 moles
O: \(\frac{61.2 g}{35.45\frac{g}{mol} }\)= 1.72 moles
The empirical formula must be expressed using whole number relationships, for this the numbers of moles are divided by the smallest result of those obtained. In this case:
P: \(\frac{0.86 moles}{0.86 moles }\)= 1
N: \(\frac{0.86 moles}{0.86 moles }\)= 1
O: \(\frac{1.72 moles}{0.86 mole}\)= 2
Therefore the P: N: O mole ratio is 1: 1: 2
Then, the empirical formula is P₁N₁O₂= PNO₂, with a empirical mass of 31 g/mol + 14 g/mol + 2× 35.45 g/mol= 115.9 g/mol
The molecular formula can be calculated as MF= n(EF)
where:
MF= molecular formulan=molecula mass÷ empirical massEF= empirical formulaIn this case, the value n can be calculated:
n= 812 g/mol÷ 115.9 g/mol
Solving:
n= 7
Then, the molecular formula can be calculated as MF= 7×EF
Finally, the molecular formula is P₇N₇O₁₄.
Learn more empirical formula and molecular formula:
brainly.com/question/26766865
brainly.com/question/13058832
#SPJ1
How much heat is required to melt 20 grams of ice? (delta Hfus of ice = 6.01kJ/mol)
Answers
6.67 kJ of heat is required to melt 20 grams of ice. To calculate the amount of heat required to melt a certain amount of ice.
we need to use the formula:
Q = n * ΔH_fus
where Q is the amount of heat required, n is the number of moles of ice, and ΔH_fus is the heat of fusion of ice.
To find the number of moles of ice in 20 grams, we need to divide the mass by the molar mass of ice. The molar mass of water is 18.015 g/mol.
n = m / M = 20 g / 18.015 g/mol = 1.110 mol
Now we can use the formula above to calculate the amount of heat required:
Q = n * ΔH_fus = 1.110 mol * 6.01 kJ/mol = 6.67 kJ
Therefore, 6.67 kJ of heat is required to melt 20 grams of ice.
To learn more about grams please click on below link.
https://brainly.com/question/11260752
#SPJ1
Is atomic radius a periodic property of atoms?
Answers
Major periodic trends include atomic radius, ionization energy, electron affinity, electronegativity, valency and metallic character.
What is the molar mass of H2CO3?
(Molar mass of H = 1.0079 g/mol; C = 12.010 g/mol; O = 15.999 g/mol)
29.02 g/mol
46.04 g/mol
62.02 g/mol
72.08 g/mol
Answers
Answer:
62.02 g/mol
62.03 g/mol
Explanation:
62.03 g/mol
The molar mass of H2CO3 is 62.02 g/mol
What is molar mass?The molar mass of the compound is the sum of molar mass of indivitual atom multiple by number of that atom.
Calculation of molar mass of H2CO3 ,
Molar mass of H2CO3 = Molar mass of H*2 + Molar mass of C + Molar mass of O*3
Molar mass of H2CO3 = 1.0079*2 + 12.010 + 15.999*3
Molar mass of H2CO3 = 2.0158 + 12.010 + 47.997
Molar mass of H2CO3 = 62.02 g/mol
To learn more about Molar mass here.
https://brainly.com/question/12127540
#SPJ3
If a photon of monochromatic light has a wavelength of 94 nm, what is its frequency (in units of 1/s)?
What is the energy of this photon (in Joules)?
What is the energy of 1 mole of these photons (in kJ/mol)?
Answers
The energy of the wave is 2.11 * 10^-18 J while the energy per mole is 3.5 * 10^-42 J/mol.
What is the energy?We know that the energy of the photon is dependent on the wavelength of the light as we know. In this case, we can see that the wavelength of the light is obtained as 94 nm. We shall now proceed to find the parameters as required in the question.
We have the following;
E = hc/λ
E = energy of the radiation
h = Plank's constant
c = speed of light
λ = wavelength
Then we have;
E = 6.6 * 10^-34 * 3* 10^8/94 * 10^-9
E = 2.11 * 10^-18 J
The energy per mole is obtained from;
2.11 * 10^-18 J/6.02 * 10^23
= 3.5 * 10^-42 J/mol
Learn more about wavelength:https://brainly.com/question/13533093
#SPJ1
What would be the new volume of the pressure on 550 mL is increased from 85 kPa to 150 kPa?1) name the law first 2) identify the given, write the equation, show work
Answers
Answer
Explanation
1) Boyle's law
2) Given:
Initial volume, V₁ = 550 mL
Initial pressure, Pr = 85 kPa
Final pressure, P₂ = 150 kPa
The Boyle's law equation to calculate fssure, P₁= 85 kPa₁₁
Final pressure, P₁
Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid with liquid ammonia. Calculate the moles of ammonia needed to produce of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to significant digits.
Answers
Answer:
The balanced chemical equation:
3 NH₃ + H₃PO₄ → (NH₄)₃PO₄
From the equation we see:
3 moles of NH₃ gives 1 mole (NH₄)₃PO₄
0.085 mole NH₃ will gives ?? mole (NH₄)₃PO₄
By cross multiplication:
number of moles of (NH₄)₃PO₄ = 0.085 * 1/3 = 0.028 mole
Explanation:
The metric prefix m would be presented as 10 to the power of:

Answers
Answer:
\(-3\)
Explanation:
Here, we want to get the metric prefix m value
This means we want to get power to which it would be raised
Mathematically,we have this as the milli
The milli refers to thousandth
From what we have here, this is the power of -3
So the prefix m represents :
\(10^{-3}\)for the following equation to be bal
Answers
Answer:
What's the rest of the question
Explanation:
How much energy is required to raise the temperature of 4.0 g of mercury metal from 9.3 oC to 83.0 oC.
Answers
From the specific heat capacity of mercury, the amount of heat energy required to raise the temperature of 4.0 g of mercury metal from 9.3 °C to 83.0 °C is 77.792 J.
What is the specific capacity of mercury?The specific heat capacity of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius or kelvin.
The specific heat capacity of a substance is a constant that can be used to calculate the amount of heat required to raise the temperature of a given mass of a substance to any temperature.
The specific heat capacity of mercury is 0.140 J/g/k.
The formula for calculating specific heat capacity is given below:
Specific heat capacity, c = Δq/mΔT
where;
Δq = heat change
m = mass of the substance
ΔT = temperature change
The Heat required, Δq, will then be:
Δq = m * c * ΔT
Heat required, Δq = 4.0 * 0.140 * (83.0 - 9.3)
Heat required, Δq = 77.792 J
Lear more about specific heat capacity at: https://brainly.com/question/26866234
#SPJ1
The temperature inside my refrigerator is about 40 Celsius. That temperature in Kelvin is K.
I place a balloon in my fridge that initially has a temperature of 220 C. This is K.
If the original volume of the balloon is 0.5 liters, what will be the volume of the balloon when it is fully cooled by my refrigerator? liters. (Round to two decimal places)

Answers
Substituting the given values, we have (0.5 L) / (220 + 273.15 K) = V₂ / (313.15 K).Solving for V₂, we get V₂ = (0.5 L) * (313.15 K) / (220 + 273.15 K).
Calculating this expression, the volume of the balloon when fully cooled by your refrigerator would be approximately 0.38 liters when rounded to two decimal places.To convert Celsius to Kelvin, we need to add 273.15 to the Celsius temperature. Therefore, the temperature inside your refrigerator of 40 degrees Celsius is equivalent to 313.15 Kelvin.Now, let's consider the ideal gas law, which states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin.Since the number of moles and pressure remain constant, we can write the equation as V₁/T₁ = V₂/T₂, where V₁ is the initial volume of the balloon, T₁ is the initial temperature, V₂ is the final volume, and T₂ is the final temperature.
for such more questions on values
https://brainly.com/question/27964828
#SPJ8
how would you confirm the presence of lead in an ore?
Answers
There are numerous ways to determine whether lead is present in an ore. Atomic absorption spectroscopy is a popular approach. With this method, an ore sample is dissolved in acid and then atomized in a flame or plasma.
The sample's atoms will absorb light at particular wavelengths that are peculiar to the element under investigation. The amount of light absorbed can be used to calculate how much lead is present in the sample. Inductively coupled plasma mass spectrometry and X-ray fluorescence spectroscopy are further techniques. It is crucial to remember that these procedures call for specialized tools and training, thus they ought to only be carried out in a lab by qualified experts.
To know more about spectrometry, here
brainly.com/question/31075363
#SPJ1
Consider the reaction
2NO(g) + O2(g) = 2NO2(g)
Suppose that at a particular moment during the reaction nitric oxide
(NO) is reacting at the rate of 0.066 M/s. (a) At what rate is NO2
being formed? (b) At what rate is molecular oxygen reacting?
Answers
Answer:
(a) Rate of formation of NO2 is also 0.066M/s
(b) Rate of reaction of O2 gas is 0.033M/s
Explanation:
(a) in one second, according to the equation,
2 moles of NO combines with 2moles of NO2.
Therefore 0.066M NO will still consume 0.066mole NO2.
(b) According to the equation,
2 moles NO consumes 1 mole O2, 0.0666M will consume 0.0333 mole O2
Solve this organic transformation....use - Br2,CCl4,KOH,CH3OH,Hg+2,diluted H2SO4, PCC,HBr,Mg,Dry ether,Na,H2,Pd,quinoline
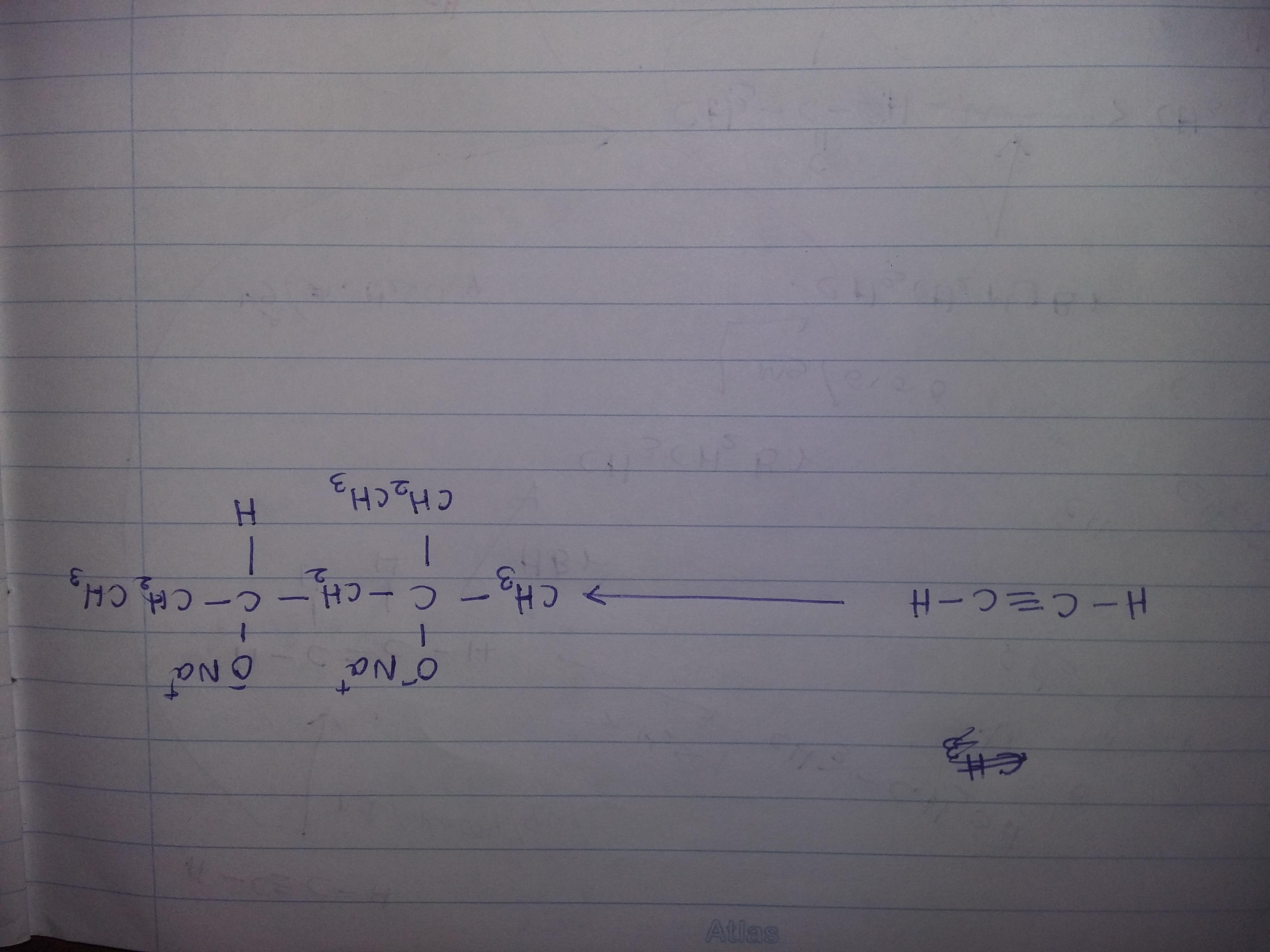
Answers
Organic transformation sequential equation using catalysts will be as follows:
2CH3-CH2-O => (alc. KOH) => CH2=CH2 + KCl + H2O => (Br2/CCl4) => CH2Br-CH2Br + Zn
CH2Br-CH2Br + Zn => (HBr /Pd) => CH2=CH2+ZnBr2
As can be visualized from above organic transformation equation, conversion of dry ether in presence of alkaline potassium hydroxide results in formation of unstable ethene. This dry unsaturated compound of ethene is stabilized by reaction that happens in presence of bromine or calcium tetrachloride as the catalyst which results in formation of ethylene bromide which in presence of highly efficient palladium as catalyst results in formation of stable ethene as byproduct. Thereby with formation of stable compound of ethylene, it releases zinc bromide as byproduct resulting completion of reaction equation. This stable product ethene is a double bonded carbon structure that is chemically extremely flammable and has planar structure.
To know more about organic transformation:
brainly.com/question/14413579
#SPJ1
How many total carbon atoms are in the structure 2 methyl, 3, 4 diethyl decane?
12
15
14
10
Answers
Answer:B
15 carbon atoms
How does electronegativity affect the polarity of the bond between two
atoms?
A. The more electronegative atom will form the positive pole of a
polar bond.
B. The more electronegative atom will form a nonpolar end of the
bond.
C. The more electronegative atom will make its end of the bond more
negative.
D. Electronegativity differences between the atoms will cancel out
bond polarity
Answers
Answer:
C. The more electronegative atom willl make its end of the
bond more negative
A P E X
The electronegativity affects the polarity of the bond between two atoms, as the more electronegative atom will make its end of the bond more negative. The correct option is C.
What is electronegativity?Electronegativity is a charge that shows the ability of an element to gain electron pairs with other elements during bonding. Electronegativity is altered by the distance between the electron and the nuclei and the atomic number of the element.
Polarity is the state of the atomic body in which it has placed charges in an opposite way to the other atoms so that they can join together.
Thus, the correct option is C. The more electronegative atom will make its end of the bond more negative.
To learn more about electronegativity, refer to the link:
https://brainly.com/question/17762711
#SPJ5
Please Help Me!!
1. What does the expression “metals eating each other” as used by the electrician refer to? Explain and give an example.
2. What are the possible electrolytes in the rusted panel?
3. Using the table for electrode potential differences (Figure 2) identify the possible composition of the screws responsible for the corrosion observed in the contact area with the copper wire.

Answers
Answer:
1. Galvanic oxidation. Example is the corrosion of aluminium wires when in contact with copper wires under wet conditions.
2. Rainwater or Damp/moist air
3. Chromium-plated steel screws or stainless steel screws or galvanized steel screws
Explanation:
1. Galvanic oxidation or corrosion occurs when two different metals with different electrode potentials are brought into contact with each other by means of an electrolyte (usually a aqueous solution), such that a redox reaction occurs leading to one metal with the more negative electrode potential (the anode) becoming oxidized, while the other less negative potential (the cathode) is reduced.
In order for galvanic corrosion to occur, three elements are required.
i. Two metals with different corrosion potentials (anode and cathode)
ii. Direct metal-to-metal electrical contact
iii. A conductive electrolyte solution (e.g. water) must connect the two metals on a regular basis.
For example oxidation (corrosion) of aluminium wires when in contact with copper wire under wet conditions.
2. The most likely electrolyte will be rainwater containing dissoved solutes (if the panel is in an exposed part of the house) or damp/moist air.
3. From the table, the most likely screw will be chromium-plated steel screws or stainless steel (made of iron and nickel) screws or galvanized steel (zinc-plated) screws.
All these possible screw components have a more negative electrode potential than copper. Thus they will serve as the anode in a galvanic oxidation with copper.
Whích kind of eclipse do you think is more special, lunar or solar?
Answers
Answer:
lunar eclipses have a nice day
The passage's author most vividly conveys the sense that Plumpp's poetry is like music when he
O uses words like "swing," "dance," and "sway" to characterize phrases in Plumpp's poems
O defines Plumpp as "the poet laureate of Chicago jazz and blues"
explains how long Plumpp has been writing about "Chicago jazz giants"
urges people to read Plumpp's poems and listen to the music Plumpp "immortalizes in print"
Answers
It is an amine, and it has less polar nitrogen-hydrogen and oxygen-hydrogen bonds.
A compound's boiling point is a physical characteristic. These intramolecular linkages between the molecules that make up a chemical affect these physical characteristics.
Alcohols and amino acids have the same kind of intermolecular linkages. The hydrogen bond is the name of this kind of bond.
The electrical attraction between a hydrogen atom from one molecule and an electronegative atom from a nearby molecule is known as a hydrogen bond.
The strength of the bond is in the following order: H.....F > H.....O > H......N
The H....N hydrogen bonds exist in amines, whereas the H....O hydrogen bonds exist in alcohols.
Consequently, the alcohol's hydrogen bonds are stronger and it will impart a higher boiling point on the compound.
Learn more about hydrogen bonds here-
https://brainly.com/question/10904296
#SPJ9
Answer:uses world like
Explanation:
The hydrogen fluoride molecule, HF, is more polar than a water molecule, H2O (for example, has a greater dipole moment), yet the molar enthalpy of vaporization for liquid hydrogen fluoride is lesser than that for water. Explain.
Answers
Answer:
Water forms more hydrogen bonds than HF
Explanation:
The answer to this question goes back to the idea of hydrogen bonding. Hydrogen bonding occurs when hydrogen is bonded to a highly electronegative atom such as fluorine or oxygen.
However, in HF, there are three lone pairs of electrons on fluorine atom and one hydrogen atom bonded to fluorine.
In H2O, there are two lone pairs of electrons on oxygen atom and two hydrogen atoms bonded to oxygen. This simply means that water can form four hydrogen bonds while HF only forms two hydrogen bonds.
This implies that H2O molecules possess more hydrogen bonding than HF molecules. Hence, the molar enthalpy of vaporization for liquid hydrogen fluoride is lesser than that for water.
Consider an ionic compound, MX3 , composed of generic metal M and generic gaseous halogen X.
The enthalpy of formation of MX3 is Δ∘f=−989 kJ/mol.
The enthalpy of sublimation of M is Δsub=129 kJ/mol.
The first, second, and third ionization energies of M are IE1=647 kJ/mol, IE2=1627 kJ/mol, and IE3=2569 kJ/mol .
The electron affinity of X is ΔEA=−369 kJ/mol . (Refer to the hint).
The bond energy of X2 is BE=153 kJ/mol.
Determine the lattice energy of MX3.
Answers
The lattice energy of the compound is -5745 kJ/mol.
What is the lattice energy?
We know that the lattice energy is the energy that is given out when a crystal lattice is formed. We obtain the energy by the use of the Hess law which would have to involve the heat that was evolved or absorbed in the process.
Lattice energy = (−989) - (129) - (153) - [(647 + 1627 + 2569)] - (−369)
Lattice energy = -5745 kJ/mol
Hence the lattice energy of the unknown compound MX3 given the data that has been provided is obtained as -5745 kJ/mol.
Learn more about lattice energy: https://brainly.com/question/18222315
#SPJ1
When 1.00 mole of NH4NO3 dissolves in water, the enthalpy change is ΔH = + 25.7 kJ. What is the enthalpy change if 17.5 grams of NH4NO3 are dissolved
Answers
Answer:
5.62 kJ
Explanation:
Let's consider the thermochemical equation for the dissolution of ammonium nitrate.
NH₄NO₃(s) ⇒ NH₄⁺(aq) + NO₃⁻(aq) ΔH° = 25.7 kJ
25.7 kJ are absorbed per 1.00 mole of NH₄NO₃. The enthalpy change when 17.5 g of NH₄NO₃ (M: 80.04 g/mol) are dissolved is:
17.5 g × 1 mol/80.04 g × 25.7 kJ/mol = 5.62 kJ
Considering the rule of three, if 17.5 grams of NH₄NO₃ are dissolved, the enthalpy change is 5.62 kJ.
In first place, the rule of three is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them.
That is, what is intended with it is to find the fourth term of a proportion knowing the other three.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied.
To solve a direct rule of three, the following formula must be followed, being a, b and c known data and x the variable to be calculated:
a ⇒ b
c ⇒ x
So: \(x=\frac{cxb}{a}\)
Being 80 g/mole the molar mass of NH₄NO₃, that is, the amount of mass that a substance contains in one mole, the number of moles that 17.5 g of the compound contain is calculated as:
\(17.5 gramsx\frac{1 mole}{80 grams} =\) 0.21875 moles
Then it is possible to apply the following rule of three: if when 1.00 mol of NH₄NO₃ dissolves in water the enthalpy change is ΔH =+25.7 kJ, when 0.21875 moles of the compound dissolves in water, the enthalpy change will have what value?
\(enthalpy change=\frac{0.21875 molesx25.7 kJ}{1 mole}\)
enthalpy change= 5.62 kJ
Finally, if 17.5 grams of NH₄NO₃ are dissolved, the enthalpy change is 5.62 kJ.
Learn more:
brainly.com/question/10936616?referrer=searchResults brainly.com/question/16487206?referrer=searchResults brainly.com/question/14446695?referrer=searchResults brainly.com/question/11564309?referrer=searchResults brainly.com/question/4025026?referrer=searchResults brainly.com/question/18650135?referrer=searchResultsH₂Se
# of Valence e" =
Lewis Structure:
Draw the Molecular Geometry
(using dashes and wedges):
Se
of e Groups =
Hybridization =
Electronic Geometry:
Molecular Geometry:
Polar or Nonpolar?
Answers
the correct answer is electrons
how many molecules of PCI5 are in 77.4g PCI5
Answers
To determine the number of molecules of PCI5, we need to use the following steps:
Convert the given mass of PCI5 to moles using the molar mass of PCI5.
Use Avogadro's number to convert the number of moles to molecules.
The molar mass of PCI5 can be calculated as follows:
P = 1 x 30.97 = 30.97
C = 1 x 12.01 = 12.01
I = 5 x 126.90 = 634.50
Molar mass of PCI5 = 30.97 + 12.01 + 634.50 = 677.48 g/mol
Mass to moles:
moles = mass / molar mass
moles = 77.4 g / 677.48 g/mol
moles = 0.114 moles
Moles to molecules:
One mole of any substance contains 6.022 x 10^23 particles (Avogadro's number).
Number of molecules = moles x Avogadro's number
Number of molecules = 0.114 mol x 6.022 x 10^23 mol^-1
Number of molecules = 6.87 x 10^22 molecules
Therefore, there are 6.87 x 10^22 molecules of PCI5 in 77.4g of PCI5.
Sir J.J. Thompson discovered the electron in 1897. How did Thompson explain the overall neutral charge of the atom?
A) Electrons travel around the nucleus at great speeds.
B) Electrons alternate between positive and negative charges.
C) Negative electrons were inside a cloud of positive charge.
D) Electrons are present all over the atom, even in the nucleus.
Answers
your answer is C you're welcome