Which idea was popularized in the United States by William Blackstone?
A. The U.S. government should be divided into three equal branches.
OB. The United States should declare its independence from Great
Britain.
C. The government does not have the right to limit a person's speech.
OD. Those accused of crimes should be considered innocent until
proven guilty

Answers
Those accused of crimes should be considered innocent until proven guilty. Hence, option D is correct.
Who was William Blackstone?Sir William Blackstone (10 July 1723 – 14 February 1780) was an English jurist, judge and Tory politician of the eighteenth century.
The main idea that was popularized in the United States by William Blackstone was that those accused of crimes should be considered innocent until proven guilty.
One of Blackstone's most famous comments was that "It is better that ten guilty persons escape than one innocent suffer."
Hence, option D is correct.
Learn more about the William Blackstone here:
https://brainly.com/question/932688
#SPJ1
Related Questions
You know that silver has a density of 10.5 g/cm3. What mass of silver will raise the level of the
water in the graduated cylinder 1.50 mL?
Answers
The mass of the silver that will raise the water by 1.5 mL is 15.75 g
What is density?The density of a substance is simply defined as the mass of the subtance per unit volume of the substance. Mathematically, it can be expressed as
Density = mass / volume
With the above formula, we can obtain the mass of the silver. Details below
How to determine the mass of the silverDensity of silver = 10.5 g/cm³Volume of water raised = 1.5 mLVolume of silver = Volume of water raised = 1.5 mL = 1.5 cm³Mass of silver =?Density = mass / volume
Cross multiply
Mass = Density × Volume
Mass of silver = 10.5 × 1.5
Mass of silver = 15.75 g
Learn more about density:
https://brainly.com/question/952755
#SPJ1
NEED HELP ASAP PLEASE AND TY

Answers
The synthesis of the ATP takes place in the organelle known as the mitochondrion. Therefore, option (A) is correct.
What is mitochondrion?Mitochondrion can be described as the membrane-bound organelles in the cytoplasm of all eukaryotic cells, that synthesize adenosine triphosphate (ATP), the main energy source used by the cell.
It is also known as the “Powerhouse of the cell,” Mitochondrion is found inside the cytoplasm and essentially functions as the cell’s digestive system.
Mitochondrion plays a major role in breaking down nutrients and producing energy-rich molecules for the cell. Many of the biochemical reactions in cellular respiration occur within the mitochondria.
The mitochondrion's structure can be described as a double-membraned, rod-shaped structure found in both animal cells and plants.
Learn more about Mitochondrion, here:
https://brainly.com/question/17091391
#SPJ1
Consider the following expression:8.30 x 10^-5 = x(0.100 + x)^2We can solve for x using a technique called successive approximations.8.30 x 10^-5 =x1 (0.100)^2Step 1: If we assume that x is very small compared to 0.100 (such that 0.100 x ? 0.100) then our first approximation of x (let\'s call it x1) can be calculated asx1 = ?Step 2: Now, take your first approximation of x and plug it into the full equation.8.30 x 10^-5 =x2 (0.100 + x1)^2x2 = ?Step 3: Each successive approximation uses the value from the previous approximation.8.30 x 10^-5 =x3 (0.100 +x2)^2x3 = ?Step 4, etc.: Continue this process until two x values agree within the desired level of precision.x4 = ?x5 = ?Which values are the first to agree to two significant figures?a. x1 and x2b. x2 and x3c. x3 and x4d. x4 and x5Which values are the first to agree to three significant figures?a. x1 and x2b. x2 and x3c. x3 and x4d. x4 and x5
Answers
Answer:
a. x3 and x4 are the first to agree to two significant figures
b. x4 and x5 are the first to agree to three significant figures
Explanation:
8.30 x 10⁻⁵ = x1(0.100)²
8.30 x 10⁻⁵ = 0.01x1
x1 = 8.30 x 10⁻⁵/0.01 = 0.0083
8.30 x 10⁻⁵ = x2(0.100 + 0.0083)²
8.30 x 10⁻⁵ = (0.01172889)x2
x2 = 8.30 x 10⁻⁵/0.01172889
x2 = 0.007076543475128
8.30 x 10⁻⁵= x3 (0.100 + 0.007076543475128)²
8.30 x 10⁻⁵ = 0.011465386162581)x3
x3 = 8.30 x 10⁻⁵/0.011465386162581
x3 = 0.007239180505832
8.30 x 10⁻⁵ = x4 (0.100 + 0.007239180505832)²
8.30 x 10⁻⁵ = (0.011500241835562) x4
x4 = 8.30 x 10⁻⁵/0.011500241835562
x4 = 0.007217239531723
8.30 x 10⁻⁵ = x5 (0.1 + 0.007217239531723)²
8.30 x 10⁻⁵ = (0.011495536452803) x5
x5 = 8.30 x 10⁻⁵/0.011495536452803
x5 = 0.007220193710904
From the above results;
a. x3 and x4 are the first to agree to two significant figures
b. x4 and x5 are the first to agree to three significant figures
A mixture of solids containing a ketone, a carboxylic acid, and an amine, are dissolved in DCM. What is the best way to begin an extraction to separate the amine from the mixture
Answers
There are different ways of extraction. The best way to begin an extraction to separate the amine from the mixture is to extract with dilute NaOH.
An acid-base extraction is often used in the extraction of carboxylic acids from the organic layer and thereafter into the aqueous layer.NaOH is known to be the most common compound that is used to convert a carboxylic acid into its more water-soluble ionic carboxylate form.
But if the mixture has a compound that you want, and that can react with NaOH, another milder base such as sodium bicarbonate is preferably used.
See full question below
A mixture of solids containing a ketone, a carboxylic acid, and an amine, are dissolved in DCM. What is the best way to begin an extraction in order to separate the carboxylic acid from the mixture?
A) Extract with dilute NaOH
B) Extract with dilute HCl
C) Extract with dichloromethane
D) Extract with water
Learn more from
https://brainly.com/question/2646724
According to the United States Food and Drug Administration, the recommended daily requirement of protein is 44 g. Express in ounces the daily requirement of protein. (1 lb = 16 oz) (1 lb = 454g)
Answers
Answer:
1.55205
Explanation:
Why is there a change in energy during chemical reactions?(1 point)
Chemical bonds are formed and broken, which absorbs energy.
Chemical bonds are formed and broken, which absorbs energy.
Energy is released by the formation of chemical bonds, and energy is absorbed when the bonds are broken.
Energy is released by the formation of chemical bonds, and energy is absorbed when the bonds are broken.
Energy is released by the breaking of chemical bonds, and energy is absorbed when the bonds are formed.
Energy is released by the breaking of chemical bonds, and energy is absorbed when the bonds are formed.
Chemical bonds are formed and broken, which releases energy.
Chemical bonds are formed and broken, which releases energy.
Answers
Energy is released by the formation of chemical bonds, and energy is
absorbed when the bonds are broken.
What is a chemical reaction?A chemical reaction involves the formation of new compounds from
reactants . It involves the formation and breaking of bonds in the
elements.
Energy is released by the formation of chemical bonds and this type of
reaction is referred to as exothermic while energy is absorbed when the
bonds are broken and is referred to as an endothermic reaction.
Read more about Chemical reaction here https://brainly.com/question/16416932
Enzyme E catalyzes the transformation of reactant A to product R as follows:
A enzyme
R, -rA =200CACE0/2+CA mol/l.min
If we introduce enzyme (CE0 = 0.001 mol/liter) and reactant (CA0 = 10 mol/liter) into
a batch rector and let the reaction proceed, find the time needed for the concentration
of reactant to drop to 0.025 mol/liter. Note that the concentration of enzyme remains
unchanged during the reaction..

Answers
It will take 1200 minutes for the concentration of reactant to drop to 0.025 mol/liter.
How to determine time?The rate of reaction is given by the following equation:
\(-r_{A} = \frac{200C_{A} C_{EO}}{2+C_{A}} \frac{mol}{liter . min}\)
where
\(-r_{A}\) = is the rate of reaction (mol/liter/min)
\(C_{A}\) = is the concentration of reactant A (mol/liter)
\(C_{EO}\) = is the concentration of enzyme E (mol/liter)
Given that \(C_{EO}\) = 0.001 mol/liter and \(C_{AO}\) = 10 mol/liter. Find the time needed for the concentration of reactant to drop to 0.025 mol/liter.
Set up the following equation and solve for t:
\(C_{AO} - C_A = -r_A t\)
Substituting the given values:
10 - 0.025 = -(200)(0.025)(0.001)t
Solving for t:
t = 1200 min
Therefore, it will take 1200 minutes for the concentration of reactant to drop to 0.025 mol/liter.
Find out more on concentration of reactant here: https://brainly.com/question/30669858
#SPJ1
Determine the subunit composition of a protein from the following information:Molecular mass by gel filtration: 200 kDMolecular mass by SDS-PAGE: 100 kDMolecular mass by SDS-PAGE with 2-mercaptoethanol: 40 kD and 60 kD
Answers
Answer:
Explanation:
The protein comprises of two 60-kD polypeptides and two 40-kD polypeptides. Each one of the 40-kD chains has a disulfide-bond, which is directly bonded to a 60-kD chain.
The 100-kD units attach noncovalently to produce a protein with a molecular mass of 200 kD.
The protein consists of 200 kD in size, and the Gel filtration doesn't affect the relationship and interaction among the various subunits in the protein.
When SDS-PAGE takes place, samples are being subjected to boiling of samples and therefore undergoing denaturation conditions. The result causes disorganization in the 100 kD units.
It implies that BME is responsible for the reduction between the R1-S-S-R2 bond between 40 kD and 60 kD to R1-SH and R2-SH, resulting in separate proteins.
However, the reducing agent (BME) main task is reducing disulfide bonds in a protein.
It takes 4 pounds of steel to make a small robot. You have 48 ounces. Do you have enough? If not what do you need?
Answers
No, 48 ounces are not enough. For making a small robot we need 64 ounces which is equal to 4 pounds.
What is pound and ounces?Pound is a unit for measuring weight. 16 ounces makes one pound.
Ounce is also a unit for measuring weight. 16 ounces is equal to 1 pound
So, for making one small robot we need 4 pounds.
1 pound = 16 ounces
4 pounds = 64 ounces
But, we have 48 ounces
We need more = 64 - 48 = 16 ounces or 1 pound
No, 48 ounces are not enough. For making a small robot we need 64 ounces which is equal to 4 pounds.
To know more about pounds, check out:
https://brainly.com/question/22599208
#SPJ1
Check all of the boxes that are true about the proton:
it is outside the nucleus
it has a positive charge
it has no mass
it has a negative charge
it is inside the nucleus
it is the same as the atomic number
it is the same as the number of neutrons
75% of the isotopes have a mass
Answers
Answer:
it is outside the nucleus F
it has a positive charge T
it has no mass F
it has a negative charge F
it is inside the nucleus ...it is part OF the nucleus.
it is the same as the atomic number T
it is the same as the number of neutrons F
75% of the isotopes have a mass ima just guess cuz i dunno about this one...i think it matters on the atom element.
Explanation:
10^-2 M NH3 in water Kb=1.8*10^-5, conc. of all species=?
Answers
1.34 x 10^{-3} is the concentration of NH4 .
What is pH ?
pH, a quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Widely used in chemistry, biology, and agriculture, this term translates the concentration value of hydrogen ions. This typically ranges from about 1 to 10-14 gram equivalents per liter. Converted to a number between 0 and 14. At neutral (neither acidic nor alkaline), the concentration of hydrogen ions is 10−7 gram equivalents per liter, which corresponds to a pH of 7. Solutions below pH 7 are considered acidic. Solutions with a pH greater than 7 are considered basic or alkaline.
To learn more about the pH , click the given link ;
https://brainly.com/question/172153
#SPJ1
Part A
How much heat is required to vaporize 28.3 g of water at 100 °C? (AHvap (H₂O) = 40.7 kJ/mol)
Express your answer with the appropriate units.
Answers
First, we have to remember the equation to calculate the heat of evaporation:
\(Q_{vap}=\text{ }\Delta H_{vap}*\text{ m}_{sust}\)Q is the heat, ΔH is the vaporization heat of the substance, and m is the mass.
If we have the vaporation heat in terms of moles (as in this case), we have to multiply it by the number of moles instead of the mass. For that purpose, we have to calculate the molecular weight of the water:
\(M.W.\text{ of water = 1*2+16=18 g/mol}\)Then, we can pass the grams to moles:
\(28.3\text{ g *}\frac{1\text{ mol}}{18\text{ g}}=1.5722\text{ moles}\)And we can finally calculate the heat:
\(Q_{vap}=\text{ 40.7 }\frac{kJ}{mol}*1.5722\text{ mol = 63.9894 kJ}\)The answer is that the necessary heat to evaporate the water is 63.9894 kJ approx.
Determine The Bond Angle Highlighted In Red For Each Given Molecule.

Answers
There are two characteristics of molecules, one is geometry and other is shape. Shape is excluding lone pair surrounding the central element and geometry is including the lone pair. Therefore, the angle of the given molecule can be found out by VSEPR theory.
What is VSEPR theory?
VSEPR stands for valence shell electron pair repulsions. VSEPR theory is used to predict the shape and geometry of molecules on the basis of valence electrons pairs that are present around the central element of the molecule.
According to VSEPR theory, Lone pair lone pair repulsion is greater than bond pair bond pair repulsion. There are so many limitations of VSEPR theory. There is a repulsion between bond pair electrons and lone pairs present on the central element.
a) bond angle is 180°
b)bond angle is 120°
c)bond angle is 107.28'
d)bond angle is 109.28'
Therefore, the angle of the given molecule can be found out by VSEPR theory.
To know more about VSEPR theory, here:
https://brainly.com/question/19582124
#SPJ1
Convert 0.50 moles of calcium nitrate into its corresponding mass in grams!
Answers
Answer:
82.0439 grams
Explanation:
1 moles Calcium Nitrate, or 164.0878 grams.
164.0878/2 = 82.0439 grams
The result of conversion 0.50 moles of calcium nitrate into its corresponding mass is 82 grams. The molar mass of calcium nitrate is 164 g/mol.
ExplanationFormula:
\(\boxed{\tt g=n\times Mr}\)
n = moles number of molecules, mol.g = mass of molecules, grams.Mr = molar mass, g/mol.Given:
n Ca(NO₃)₂ = 0.5 moles.From periodic table:Ar Ca = 40 g/mol.
Ar N = 14 g/mol.
Ar O = 16 g/mol.
Therefore,
First, calculate the molar mass of calcium nitrate.
Mr Ca(NO₃)₂ = Ar Ca + 2 x Ar N + 2 x 3 x Ar OMr Ca(NO₃)₂ = 40 + 2 x 14 + 6 x 16
Mr Ca(NO₃)₂ = 40 + 28 + 96
Mr Ca(NO₃)₂ = 164 g/mol.
Second, calculate the mass of calcium nitrate.
g = n × Mrg = 0.50 moles × 164 g/mol g = 82 gSo, 0.50 moles of calcium nitrate correspond to a mass of 82 grams.
Learn more about other mass calculation of some molecules on:
https://brainly.com/question/30864418https://brainly.com/question/30864555https://brainly.com/question/30864550Calculate the mass in grams of each of the followinga. 5.94 x 10^20 H2O2 moleculesb. 2.8 x 10^22 SO2 moleculesc. 4.5 x 10^25 O3 moleculesd. 9.85 x 10^19 CH4 molecules
Answers
Answer:
\(0.0335\ \text{g}\)
\(2.978\ \text{g}\)
\(3586.84\ \text{g}\)
\(0.0026\ \text{g}\)
Explanation:
Mass in grams is given by
\(m=\dfrac{nM}{N_A}\)
where
n = Number of molecules
\(N_A\) = Avogadro's number = \(6.022\times 10^{23}\ \text{mol}^{-1}\)
M = Molar mass of molecule
Molar mass of \(H_2O_2\) = 34.0147 g/mol
\(n=5.94\times 10^{20}\)
\(m=\dfrac{5.94\times 10^{20}\times 34.0147}{6.022\times 10^{23}}\\\Rightarrow m=0.0335\ \text{g}\)
Mass of \(H_2O_2=0.0335\ \text{g}\)
Molar mass of \(SO_2\) = 64.066 g/mol
\(n=2.8\times 10^{22}\)
\(m=\dfrac{2.8\times 10^{22}\times 64.066}{6.022\times 10^{23}}\\\Rightarrow m=2.978\ \text{g}\)
Mass of \(SO_2=2.978\ \text{g}\)
Molar mass of \(O_3\) = 48 g/mol
\(n=4.5\times 10^{25}\)
\(m=\dfrac{4.5\times 10^{25}\times 48}{6.022\times 10^{23}}\\\Rightarrow m=3586.84\ \text{g}\)
Mass of \(O_3=3586.84\ \text{g}\)
Molar mass of \(CH_4\) = 16.04 g/mol
\(n= 9.85\times 10^{19}\)
\(m=\dfrac{9.85\times 10^{19}\times 16.04}{6.022\times 10^{23}}\\\Rightarrow m=0.0026\ \text{g}\)
Mass of \(CH_4=0.0026\ \text{g}\)
The activation energy, Ea, for a particular reaction is 37.8 kJ/mol. If the rate constant at 280 K is 0.178 M/s, then what is the value of the rate constant at 381 K? (R = 8.314 J/mol • K)
Answers
The rate constant that we have at 381 K will be 2.19 M/s.
What is the Arrhenius equation?The Arrhenius equation suggests that the rate of a reaction increases with temperature, because higher temperatures provide more kinetic energy to the reactant molecules, making them more likely to react.
By the use of the Arrhenius equation, we have that;
ln k2/k1 = -Ea/R(1/T2 - 1/T1)
ln k2/0.178 = -37.8 * 10^3/8.314 (1/381 - 1/280)
ln k2/0.178 = - 4647 * (2.62 - 3.57) * 10^-3
lnK2 = 0.786
k2 =e^0.786
k2 = 2.19 M/s
Learn more about Arrhenius equation:https://brainly.com/question/12907018
#SPJ1
Complete the statements by writing the number
from the graph.
The substance is in the gas phase only in region
The substance is in both the liquid and the solid
phase in region
The substance is in only the liquid phase in region
The melting point is the temperature at region
The boiling point is the temperature at region
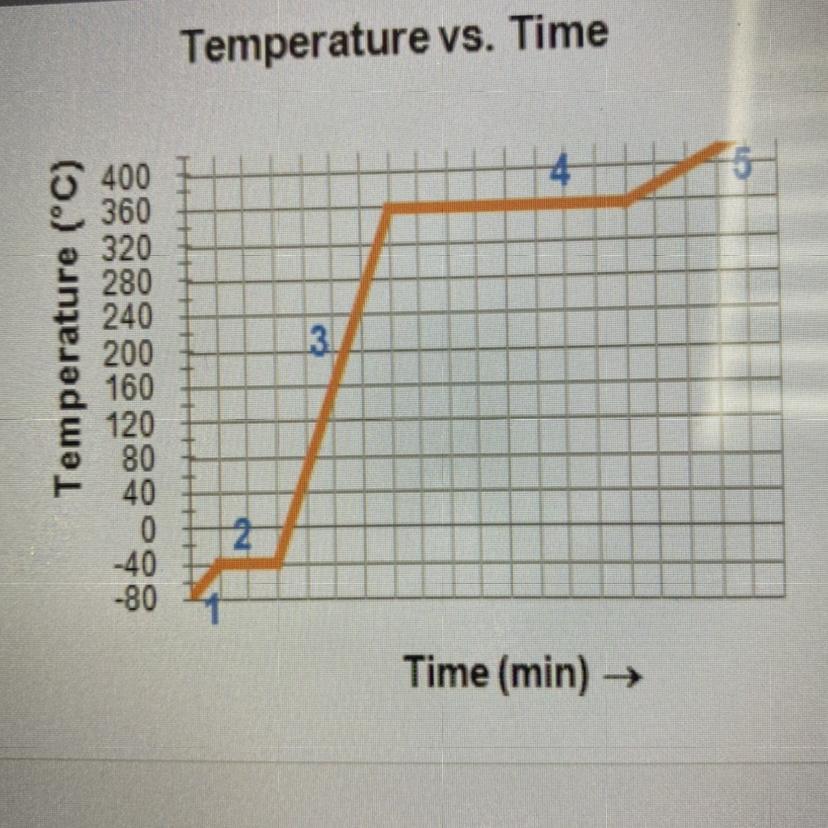
Answers
Answer :
The substance is in the gas phase only in region → 5
The substance is in both the liquid and the solid phase in region → 2
The substance is in only the liquid phase in region → 3
The melting point is the temperature at region → 2
The boiling point is the temperature at region → 4
Explanation :
Six phases of substance:
Melting or fusion : In this process the phase changes from solid state to liquid state at constant temperature.Freezing : In this process the phase changes from liquid state to solid state at constant temperature.Evaporation : In this process the phase changes from liquid state to gaseous state at constant temperature.Condensation : In this process the phase changes from gaseous state to liquid state at constant temperature.Sublimation : In this process the phase changes from solid state to gaseous state without passing through the liquid state at constant temperature.Deposition : In this process the phase changes from gaseous state to solid state without passing through the liquid state at constant temperature.Answer:
5, 2, 3, 2, 4.
Explanation:
got it correct on edge 2021
hexanol to sodium hexanoate
Answers
Answer:
Molecular Formula: C6H11NaO2
Parent Compound: CID 8892 (Hexanoic acid)
Explanation:
When a mixture of 1−hexanol and hexanoic acid in diethyl ether is shaken with an aqueous NaHCO 3 solution, Hexanoic acid will react with NaHCO 3 to form sodium hexanoate.
CH 3 −CH 2 −CH 2 −CH 2 −CH 2−COOH+NaHCO 3→CH 3−(CH 2 ) 4 −COONa+H 2 O+CO 2
the work function of magnesium metal is 5 86/10J
a, calculate the minimum frequency of required to release elections from the metal.
b, calculate the kinetic energy of the ejected electronic light of frequency 2.00/10 s is used to irradiating the metal.
Answers
a) To calculate the minimum frequency of electromagnetic radiation required to release electrons from the metal, you can use the following formula:
f = W / h
where f is the minimum frequency of electromagnetic radiation required, W is the work function of the metal in joules, and h is the Planck constant in joules per second.
Plugging in the values for W and h, you get:
f = (5.86 x 10^-19 J) / (6.626 x 10^-34 J/s) = 8.9 x 10^14 Hz
This is the minimum frequency of electromagnetic radiation required to release electrons from the magnesium metal.
b) To calculate the kinetic energy of the ejected electronic light of frequency 2.00 x 10^14 Hz, you can use the following formula:
KE = hf - W
where KE is the kinetic energy of the ejected electron, h is the Planck constant in joules per second, f is the frequency of the electromagnetic radiation in hertz, and W is the work function of the metal in joules.
Plugging in the values for h, f, and W, you get:
KE = (6.626 x 10^-34 J/s) * (2.00 x 10^14 Hz) - (5.86 x 10^-19 J) = 1.32 x 10^-19 J - 5.86 x 10^-19 J = -4.54 x 10^-20 J
This is the kinetic energy of the ejected electron when light of frequency 2.00 x 10^14 Hz is used to irradiate the magnesium metal. Since the kinetic energy is negative, this means that the electron is not released from the metal when irradiated with this frequency. The frequency of the electromagnetic radiation needs to be higher than the minimum frequency required to release the electron in order for the electron to be ejected from the metal.
The lab stockroom has a bottle of Sulfuric acid labeled 15.5M. How many mL of water will you need to add to 5.41mL of the acid solution in order to get a concentration of 0.175M?
Answers
The volume of water needed to make the solution is 473.76 mL
We'll begin by calculating the volume of the diluted solution.
Molarity of stock solution (M₁) = 15.5 MVolume of stock solution needed (V₁) = 5.41 mL Molarity of diluted solution (M₂) = 0.175 MVolume of diluted solution (V₂) =?M₁V₁ = M₂V₂
15.5 × 5.41 = 0.175 × V₂
83.855 = 0.175 × V₂
Divide both side by 0.175
V₂ = 83.855 / 0.175
V₂ = 479.17 mL
Finally, we shall determine the volume of water needed.
Volume of stock solution needed (V₁) = 5.41 mLVolume of diluted solution (V₂) = 479.17 mLVolume of water =?Volume of water = V₂ – V₁
Volume of water = 479.17 – 5.41
Volume of water = 473.76 mL
Therefore, the volume of the water needed is 473.76 mL
Learn more about dilution: https://brainly.com/question/16167657
using a cutting board to cut raw chicken and then using the same cutting board to cut fresh fruit with only a rinse is between
Answers
Answer:
its is wrong to do that
Explanation:
the smell of the meat will mix with that of the fruits
Which of the following is an example of chemical weathering?
A . Rock grinded away by other rocks in a stream bed
B. Ice freezing and thawing in cracks
C. Waves beating on the shoreline
D. Dissolving of rock due to acid rain
Answers
Answer:
D. Dissolving of rock due to acid rain.
Dihydrogen dioxide decomposes into oxygen gas and water. How many moles of Dihydrogen dioxide are required to produce 6.38 moles of oxygen?
Answers
Answer
12.76 moles H₂O₂
Explanation
Given:
Moles of oxygen produced = 6.38 moles
What to find:
The moles of Dihydrogen dioxide required to produce 6.38 moles of oxygen.
Step-by-step solution:
Step 1: Write the balanced equation for the decomposition reaction.
2H₂O₂ --------> O₂ + 2H₂O
Step 2: Calculate the moles of H₂O₂ required.
From the balanced equation;
2 mol H₂O₂ produced 1 mol O₂
x mol H₂O₂ is required to produce 6.38 mol O₂
Cross multiply and divide both sides by 1 mol O₂.
\(x=\frac{6.38mol\text{ }O₂}{1mol\text{ }O₂}\times2mol\text{ }H₂O₂=12.76\text{ }mol\text{ }H₂O₂\)The moles of Dihydrogen dioxide required to produce 6.38 moles of oxygen = 12.76 moles H₂O₂
8 x10^23 atoms Na to moles
Answers
Answer
Moles of Na = 1.328 moles
Explanation
Given:
Atoms of Na = 8 x10^23 atoms
Required: number of moles of Na
Solution
1 mole = 6.022x10^23 atoms
x moles = 8 x10^23 atoms
solve for x
x = (8 x10^23 atoms x 1 mole)/6.022x10^23 atoms
x = 1.328 moles
The chief advantage of the metric system over other system of measurement is that it
Answers
Answer:
The metric system goes by powers of ten, so it's very easy to measure. That would be the main advantage, measurements of ten. We can also say it's the most used measurement around the world, so all scientists have little to no conversion, but the main answer is probably the first one :)
An atom with 14 protons, 14 neutrons, and 16 electrons is stable, -2 charge
stable, +2 charge
unstable, -2 charge
unstable, no charge *
Answers
We can see that an atom with 14 protons, 14 neutrons, and 16 electrons is unstable, and has a -2 charge.
So the correct option is the third one.
What can we say about the atom?An atom with 14 protons, 14 neutrons, and 16 electrons is not stable. The number of protons in an atom, also known as its atomic number, determines its element and its chemical properties. In this case, the atom has 14 protons, which corresponds to the element silicon (Si) on the periodic table.
For an atom to be stable, it should have a balanced number of protons and electrons. Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or electron shells. The number of electrons in a stable atom should be equal to the number of protons, resulting in a neutral charge overall.
In this case, the atom has 14 protons and 16 electrons, which means it has two more electrons than protons, resulting in a net charge of -2. This is an example of an ion.
Learn more about atoms.
https://brainly.com/question/17425565
#SPJ1
Which of the following best defines crustal deformation? the constructive force of hot molten rock from the mantle that reaches Earth's surface, resulting in new landforms the outermost rocky layer of Earth the constructive force that moves sediments from one place and lays them to rest at another, forming landforms the constructive force that is the result of the edges of Earth's crust pushing and pulling against each other
Answers
The best definition of crustal deformation is that it is the constructive force that is the result of the edges of Earth's crust pushing and pulling against each other.
What is crustal deformation?Crustal deformation refers to the changes in the shape, position, and orientation of the Earth's crust due to the forces acting on it.
These forces can be compressional, tensional, or shear, and they cause the crust to buckle, fold, fault, and uplift.
Crustal deformation can result in the formation of new landforms, such as mountains, valleys, and plateaus, and can also cause earthquakes and volcanic eruptions.
Thus, the best definition of crustal deformation is that it is the constructive force that is the result of the edges of Earth's crust pushing and pulling against each other.
More on crustal formation can be found here: https://brainly.com/question/13490737
#SPJ1
What is the molar mass of magnesium sulfate, MgSO4?
Answers
Answer:
120.37 g/mol is the molar mass of magnesium sulfate
Which type of molecule is acetone?
A. Amine
B. Ketone
C. Alcohol
D. Aldehyde
Answers
Answer: B. Ketone
Explanation: Another name for acetone is propanone, and it is the main ketone as it has the functional group in the middle, a CH3 on each side. Ketones have the C=O functional group.
A muffi n recipe calls for cream of tartar, or potassium
hydrogen tartrate, KHC4H4O6(s). Th e amount of
cream of tartar that is required contains 2.56 × 1023
atoms of carbon. What amount in moles of
potassium hydrogen tartrate is required?
Answers
A muffi n recipe calls for cream of tartar, or potassium hydrogen tartrate. The amount of cream of tartar that is required contains 2.56 ×10²³atoms of carbon. 0.42moles of potassium hydrogen tartrate is required
In the Global System of Units (SI), the mole represents the unit of material quantity. How many fundamental entities of a particular substance are present within an object a sample is determined by the quantity of that material. An elementary entity can be a single atom, a molecular structure, an ion, a charged particle pair, or a particle that is subatomic like a proton depending on the makeup of the substance.
For instance, despite the fact that the two substances have different volumes and masses, 10 moles of water because 10 moles of the chemical element mercury both contain the same quantity of stuff, because the mercury comprises exactly one particle for each molecule of water.
mole = 2.56 ×10²³/ 6.022×10²³
= 0.42moles
To know more about mole, here:
https://brainly.com/question/26416088
#SPJ1