Which feature of a molecule can be determined from its NMR spectrum?
Answers
Among the features that can be determined from its NMR spectrum are: number of distinct proton environments, relative number of protons in each environment, chemical shift values for each environment, and splitting patterns.
The proton (1H) nuclear magnetic resonance (NMR) spectrum of a molecule helps to identify several features of that molecule.
The number of distinct proton environments is equivalent to the number of different kinds of protons in the molecule. For example, in a molecule with three different types of protons, such as \(CH3CH2OH\) (ethanol), there will be three separate peaks in the spectrum.
The relative number of protons in each environment, which is proportional to the area under each peak, provides information on the molecule's composition.
The chemical shift values for each environment are a measure of the strength of the magnetic field felt by the protons in that environment. The magnetic field strength is influenced by the neighboring atoms and functional groups.
Therefore, the chemical shift can be used to infer information about the molecule's electronic structure and functional groups. The splitting patterns in a peak provide information about the number of neighboring protons. This helps to infer the connectivity of the molecule.
To know more about NMR spectrum refer to-
brainly.com/question/30546657#
#SPJ11
Related Questions
what is precipitating ???
anyone please tell me how to delete an I'd
Answers
Explanation:
Desposition of solid matter in a solution
in the transition state for this compound, the negative charge is divided between the leaving cl atom and the incoming br atom. based on the hammond postulate, how would you expect that charge to be distributed between the two atoms?
Answers
Bimolecular nucleophilic substitution (SN2) reactions are concerted reactions in which both the nucleophile and the substrate are involved in the rate-limiting step. Bonds are broken and new bonds are formed in a single step thanks to the coordinated nature of this reaction. In order to comprehend this reaction, it is essential to examine the transition state, which replicates the concerted rate-limiting step.
What is Hammond postulate?
The geometrical structure of the transition state of an organic chemical reaction is described by Hammond's postulate (also known as the Hammond-Leffler postulate), a physical organic chemistry hypothesis.
According to the postulate, which George Hammond first put forth in 1955, if two states, such as a transition state and an unstable intermediate, happen concurrently during a reaction process and have nearly the same amount of energy, their interconversion will only cause a slight reorganization of molecular structures.
Bimolecular nucleophilic substitution (SN2) reactions are concerted reactions in which the rate-limiting step involves both the nucleophile and the substrate. Due to the concerted nature of the reaction, the bonds are broken and new bonds are formed all in one step. It is crucial to examine the transition state, which resembles the concerted rate-limiting step, in order to interpret this reaction. The halide (L) bond is broken while the nucleophile forms a new bond with the carbon in the "Depiction of SN2 Reaction" figure.
To learn more about charge to be distributed, click on the below link:
https://brainly.com/question/14799724
#SPJ4

Chalk is a silicate carbonate evaporite sandstone QUESTION 33 a photosyntehtic creature with a silica shell can be a O coccolithophorid foraminifer diatom radiolarian QUESTION 34 recrystallization of chalk at the ocean bottom (not in metamorphic conditions) can give us O micrite chert marble quartzite
Answers
Diatoms are single-celled algae that have a silica (silicate) shell called a frustule.
Diatoms are photosynthetic organisms and are known for their intricate and diverse shapes. Diatoms are commonly found in freshwater and marine environments and play a significant role in the global carbon cycle.
Micrite is a fine-grained carbonate sedimentary rock composed of tiny carbonate particles. It forms through the precipitation and accumulation of carbonate minerals, such as calcite or aragonite, in marine environments. In the case of chalk, which is primarily composed of microscopic fragments of calcium carbonate from marine organisms, recrystallization can occur at the ocean bottom under specific conditions, leading to the formation of micrites.
Therefore, it's important to note that chert, marble, and quartzite are not the typical products of recrystallization of chalk at the ocean bottom.
For more details regarding diatoms, visit:
https://brainly.com/question/11446176
#SPJ4
Which number indicates the energy level of an atom's valence electrons?
O A. The period number
O B. The group number
O c. The atomic number
O D. The mass number
Answers
Answer:A, The Period Number
Explanation:
just took the test
what is meant bt absolute 0 temperature?
Answers
Absolute zero is an idea in thermodynamics that describes the lowest energy system.
Explanation:Thermodynamics
Thermodynamics is the study of thermal energy and heat. One of the most important ideas in thermodynamics is entropy. Entropy is defined as the chaos within a system. Chaos is the movement or different orientations of molecules in a system. Entropy is related to temperature; the higher the temperature, the higher the entropy. High entropy means there is a large amount of chaos in a system, while low entropy is low chaos.
Absolute zero
Absolute zero occurs when the temperature of a system is 0 kelvin or -273.15 °C. Zero kelvin is the theoretical temperature in which entropy equals 0. This means that there is no movement or energy within a system. Absolute zero is the lowest energy a system can possibly have.
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11
if an element forms 1- ion, in which group of the periodic table would you expect to find it
Answers
Many of the elements on the periodic table will always form ions that have the same charge. The alkali metals (shown in yellow) always form +1 ions. The alkaline earth metals (red) always form +2 ions. The halogens (blue) always form -1 ions.
Answer:
the correct answer is 17
The balanced net ionic equation for precipitation of CaCO3 when aqueous solutions of Li2CO3 and CaCl2 are mixed is
Answers
Answer:
Li2CO3+CaCl2=CaCO3+LiCl
5.00 mL of base is added to the 15.00 mL of acid in previous problem what is the ph of the resulting solution containing 0.0002 moles of hydrogen ions
Answers
The pH of the resulting solution containing 0.0002 moles of hydrogen ions and a total volume of 20.00 mL is approximately 2.30.
To calculate the pH of the resulting solution, we first need to determine the concentration of hydrogen ions (H+) in the solution.
Since there are 0.0002 moles of H+ ions in a total volume of 20.00 mL (15.00 mL acid + 5.00 mL base), we can find the concentration by dividing the moles of H+ ions by the total volume in liters (0.02 L).
The concentration of H+ ions is 0.0002 moles / 0.02 L = 0.01 M.
Next, we can use the pH formula: pH = -log[H+].
By plugging in the concentration of H+ ions, we get pH = -log(0.01) which results in a pH of approximately 2.30.
Learn more about hydrogen ions here:
https://brainly.com/question/20309096
#SPJ11
Is lotion pure or impure?
Answers
Answer:
Pure
Explanation:
Lotion is pure. Hope this helps!
Answer:
I think pure
hope this helps
have a good day :)
Explanation:
What mass of potassium nitrate is found in 542.41 mL of a 0.89 M aqueous sodium chloride solution? The molar mass of potassium nitrate is 101.10 g/mol.
Answers
Answer:
24.36 grams.
Explanation:
Hydroxyapatite, Ca₅(PO₄)₃(OH), is the main mineral component of dental enamel, dentin, and bone. Coating the compound on metallic implants (such as titanium alloys and stainless steels) helps the body accept the implant. When placed in bone voids, the powder encourages natural bone to grow into the void. Hydroxyapatite is prepared by adding aqueous phosphoric acid to a dilute slurry of calcium hydroxide. (a) Write a balanced equation for this preparation.
Answers
Ca5(PO4)3(OH) + 9H2O →5Ca(OH)2 + 3H3PO4.
According to the information given, 135.62 g of hydroxyapatite were produced.
What is hydroxyapatite ?Calcium apatite has the chemical formula Ca5(PO4)3(OH), however it is most commonly written Ca10(PO4)6(OH)2 to indicate that the crystal unit cell consists of two entities. Hydroxyapatite, also known as hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite. The complex apatite group's hydroxyl endmember is called hydroxyapatite. Fluorapatite or chlorapatite can be created by substituting fluoride, chloride, or carbonate for the OH ion. It forms a hexagonal crystal structure when it crystallizes.
Human bone contains up to 50% by volume and 70% by weight of bone mineral, a modified form of hydroxyapatite.
To learn more about hydroxyapatite from the given link:
brainly.com/question/27191380
#SPJ4
What does the acronym BARF
Answers
Answer:
Born Again Raw Feeders
Pls help me its due in 20 minutes.

Answers
Answer:
11. 1, 4.5
12. right; 4.5 > 1
13. 10, 3
14. left; 10 > 3
Explanation:
All you're being asked to do is count the squares in the pictures.
11. The number of squares for the left wave from bottom to top is 2, so the amplitude is 1/2 that, 2/2 = 1. For the right wave it is 9 squares from bottom to top, so the amplitude is 9/2 = 4.5.
left amplitude: 1, right amplitude: 4.5
__
12. The right wave has greater amplitude. Evidence: 4.5 > 1. It also takes up more vertical space in the picture.
__
13. It is difficult to determine the wavelength of the left wave, because two full peaks are not shown. If we try to match corresponding points, they appear to be about 10 squares apart.
The right wave has 2 peaks in 6 squares, so about 3 squares between peaks.
left: wavelength 10, right: wavelength 3
__
14. The left wave has greater wavelength. Evidence: 10 > 3.
Answer:
11. 1, 4.5
12. right; 4.5 > 1
13. 10, 3
14. left; 10 > 3
Explanation:
PLEASE URGENT HELP!! 30 POINTS!!!!!!

Answers
Answer:
CH³–C=CH——CH³–CH=CH²
Explanation:
THIS IS PROPYNE TO PROPENE
can someone please help me with 1 & 2

Answers
As a result, when 1.5 moles of H2 and 1.5 moles of Cl2 react, 3 moles of HCl would be created.
The number of HCl molecules required to balance the equation Mg + HCl mgcl2 H2 is given?2 should be expressed as a co-efficient of the hydrogen atoms present on the reactant side in order to balance the hydrogen atoms. Meaning that both the product and reactant sides now contain two hydrogens is Mg + 2 HCl MgCl 2 + H 2. As a result, the equation is now balanced.
The reaction's chemical equation is as follows:
H2 + Cl2 → 2HCl
According to the equation, for every 1 mole of H2 that reacts, 1 mole of Cl2 is required, and 2 moles of HCl are produced.
Therefore, for 1.5 moles of H2 and 1.5 moles of Cl2, the limiting reactant would be H2, since it is completely used up in the reaction.
So, 1.5 moles of H2 would produce 2 x 1.5 = 3 moles of HCl.
To know more about moles visit:-
https://brainly.com/question/26416088
#SPJ1
The texture of many foods is determined by the physical state of the lipid phase. Which one of these statements is NOT true? a. The solid fat content versus temperature profile plays an important role in determining the texture
b. The morphology of the crystals formed plays an important role in determining the texture
c. The texture of foods containing partially crystalline lipids can be described as "plastic"
d. The polymorphic form of fat crystals is in a glassy state
Answers
d. The polymorphic form of fat crystals is in a glassy state is NOT true.
The statement that the polymorphic form of fat crystals is in a glassy state is not true. Polymorphism refers to the ability of a substance to exist in multiple crystal structures or forms. Lipids, including fats, can exhibit polymorphism, meaning they can crystallize in different arrangements or crystal forms.
When it comes to the texture of foods containing lipids, the polymorphic form of fat crystals does play a significant role. The specific crystal form and arrangement of the lipids can affect the texture of the food, influencing factors such as mouthfeel, creaminess, and stability.
The solid fat content versus temperature profile is an essential factor in determining texture, as stated in option a. The morphology of the crystals formed, as mentioned in option b, also plays a crucial role in texture. Option c is true as well, as foods containing partially crystalline lipids can exhibit a "plastic" texture.
However, option d is not accurate because the polymorphic form of fat crystals can exist in various states, including crystalline and semi-crystalline states, but not in a glassy state. Glassy states are typically associated with amorphous materials rather than crystalline structures.
To learn more about lipids, here
https://brainly.com/question/14915606
#SPJ4
When a species cannot respond to environmental changes, members of that species begin to die. When the last member of a species dies, the species becomes _________.
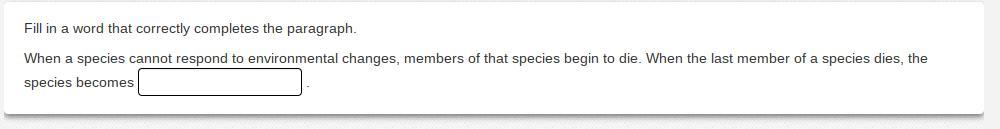
Answers
Extinct
When all of the members of species die out it is called extinct
a) which of the following reagents would oxidize cr to cr2 , but not sn to sn2 ?
Answers
To determine a reagent that can oxidize Cr to Cr2 but not Sn to Sn2, we need to consider the reduction potentials (also known as redox potentials) of these elements. The reduction potential indicates the tendency of a species to gain electrons and undergo reduction.
In this case, we want a reagent that has a higher reduction potential than Cr but a lower reduction potential than Sn. By doing so, the reagent will be able to oxidize Cr (acting as an oxidizing agent) but not Sn since Sn's reduction potential is higher than that of the reagent. Based on this criterion, one suitable reagent is acidified potassium dichromate(VI) (K2Cr2O7/H2SO4).
K2Cr2O7/H2SO4 is a commonly used oxidizing agent in various chemical reactions. In an acidic medium, it undergoes reduction and transfers oxygen atoms to the element being oxidized. The relevant reaction for this scenario can be represented as follows:
3Cr + K2Cr2O7 + 8H+ → 4Cr2+ + 2K+ + 7H2O
In this reaction, Cr is oxidized from the +3 oxidation state to the +6 oxidation state, forming Cr2+. However, the acidified potassium dichromate(VI) reagent does not possess sufficient strength to oxidize Sn. The reduction potential of Sn is lower than that of Cr, making it less prone to undergo oxidation by this reagent.
It is worth noting that the selection of appropriate reagents for oxidation or reduction reactions depends on the specific requirements of a reaction. Other reagents with higher reduction potentials than Cr, such as potassium permanganate (KMnO4), could also be considered as alternatives. However, the acidified potassium dichromate(VI) reagent mentioned above is a widely used and effective choice for oxidizing Cr without affecting Sn.
Remember to always consult reliable sources, reference materials, or your teacher to ensure the accuracy and relevance of specific reagents for a given reaction, as the field of chemistry is vast and continually advancing.
Click the below link, to learn more about oxidize Cr:
https://brainly.com/question/12947859
#SPJ11
How many grams of lead would be required to make 119 grams of water
Answers
Answer:
684
Explanation:
Mole measure the number of elementary entities of a given substance that are present in a given sample. Therefore, 684 g of lead would be required to make 119 grams of water.
What is mole?The SI unit of amount of substance in chemistry is mole. The mole is used to measure the quantity or amount of substance. We know one mole of any element contains 6.022×10²³ atoms which is also called Avogadro number.
The balanced equation for the given reaction can be written as below
Pb + PbO\(_2\) + 2H\(_2\)SO\(_4\) \(\rightarrow\)2PbSO\(_4\) + 2H\(_2\)O
Mathematically,
The mole ratio between lead and water is 1:2
(119 g H2O) / (18.01532 g H2O/mol) x (1 mol Pb / 2 mol H2O) x (207.21 g Pb/mol) = 684 g Pb
The mass of lead comes out to be 684 g.
Therefore, 684 g of lead would be required to make 119 grams of water.
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ2
Why do covalent compounds tend to be squishy?
Answers
Answer:
Since they're easy to separate, covalent compounds have low melting and boiling points. 2) Covalent compounds are soft and squishy (compared to ionic compounds, anyway). The reason for this is similar to the reason that covalent compounds have low melting and boiling points. When you hit an ionic compound with something, it feels very hard
Explanation:
mark brainliest plz
TRUE OR FALSE : The aim of an experiment can often tell us which variable is the independent variable and which variable is the dependent variable
Answers
Answer:
True
Explanation:The whole point of and expirement is to test the indepedent variable and the outcom or what your measuring is the dependent
sorry for the tone its late where i live
Which compound in 1 mol/dm3 solution has the highest PH value? a) Ethanoic acid b) Sodium chloride c) Sodium hydroxide d) Hydrogen chloride
Answers
Answer:
Explanation:
A. Ethanoic acid-acetic acid being weak acid dissocated weekly and turns solution to weakly acidic, ph will be between 4-7,
B. Hydrochloric acid - the solution would be acidic- with lots of H+ ions, Ph will be between 1-4,
C. Sodium chloride - solution prodcued netural Na+ and Cl- hence wont affect ph of solution will be equalt to 7.
D. Sodium hydrogen carbonate-NaHCO3 is weak base and the PH will be above 7,
How would I balance this?

Answers
The balanced chemical equation is as follows:
4KClO₃ ₍s₎ ⇒ 3KClO₄ ₍s₎ + 3KCl
What is balance chemical equation ?The term balanced chemical equation is defined an equation where the number of atoms of each type in the reaction is the equal on both reactants and product sides.
The chemical equation must be balanced in order to obey the law of conservation of mass. When the number of different atoms of elements in the reactants side equals the number of atoms in the products side, the chemical equation is balanced.
Thus, The balanced chemical equation is as follows:
4KClO₃ ₍s₎ ⇒ 3KClO₄ ₍s₎ + 3KCl
To learn more about the balanced chemical equation, follow the link;
https://brainly.com/question/28294176
#SPJ1
Which of the following could be produced by hydrolysis of an imine or an enamine? Select all that apply. A) Alkene B) Amine C) Ketone D) Aldehyde E) Alcohol.
Answers
The hydrolysis of an imine or an enamine can produce the following:
B) Amine
C) Ketone
D) Aldehyde
What is hydrolysis of an imine ?An imine's or an enamine's hydrolysis is a reversible process. The amine and the carbonyl molecule (aldehyde or ketone) are the end products of the process. Acid or base is used to catalyze the process.
The mechanism of the reaction is as follows:
An imine or an enamine is created when the carbonyl molecule interacts with the amine.The carbonyl compound and the amine are produced by hydrolyzing the imine or enamine.In organic chemistry, the hydrolysis of an imine or enamine is a typical reaction. It is used to make carbonyl compounds and amines.
Learn more about An imine's here : brainly.com/question/30089042
#SPJ4
AgCl is found to have 78.1% ionic character, and its gas phase dipole moment is 11.5 D. What is the distance between the Ag and Cl atoms in gaseous AgCl in picometers?
Answers
Since the dipole moment of gaseous AgCl is 11.5 D and the ionic character of AgCl is 78.1%, we can assume that the magnitude of the partial charge is 0.781.
What is magnitude ?Magnitude is a measure of the size, strength, or intensity of something. It can refer to physical objects, such as earthquakes, or abstract concepts, such as numbers or emotions. In physical sciences, magnitude is typically used to measure an object's size, intensity, or speed. In mathematics, magnitude is often used to indicate the size of a number, usually expressed as the absolute value. Magnitude is also used to describe the size of an emotion or feeling, such as happiness or sadness.
To learn more about magnitude
https://brainly.com/question/30560692
#SPJ1
What is the mass of 4.32 mol GaI3?
455 g
1,230 g
1,720 g
1,950 g
Answers
Answer:
D. 1,950 g
Explanation:
I completed the test and it is the correct answer!
The mass of one mole of gallium iodide GaI₃ is 449.7 g/mol. Hence, the mass of 4.32 moles of GaI₃ is 1950 g.
What is gallium iodide?Gallium iodide is a covalent compound formed by the electron sharing between Ga and three iodine atoms. Ga has three valence electrons and each is shared with three iodine and each iodine in turn shares 3 electrons to Ga.
Atomic mass of Ga = 69 g/mol
Atomic mass of I = 126.9 g/mol
mass of 3 iodine atoms = 3 × 126.9 = 380.7 g
Molar mass of GaI₃ = 380.7 + 69 = 449.7 g.
Molar mass of a compound is the mass of one mole of the compound. Hence, one mole of the compound is 449.7 g. Mass of 4.32 moles is :
4.32 × 449.7 ≅1950 g.
Therefore, the mass of 4.32 moles of GaI₃ is approximately 1950 g.
To find more on gallium iodide, refer here:
https://brainly.com/question/13745972
#SPJ2
What is the mass of 5 mL. of a solution if the density of the solution is 1.5 g/mL.? Show work.
Answers
Answer:
The answer is 7.5 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
volume = 5 mL
density = 1.5 g/mL
We have
mass = 1.5 × 5
We have the final answer as
7.5 gHope this helps you
3x - 12 = 7x + 8
-4x - 12 = 8
-4x - 20
X = -5
Answers
Answer:
1.Set your equations Raquel to each other
2.given
3.subtract 7x from both sides
4. Add 12 to both sides
5.divide both sides by four and you get negative 5
Explanation:
Which one of the following is a pure substance? A. Sea water B. sodium C. smoke and D. Soft drink
Answers
Answer:
B. Sodium
Explanation:
The composition of sea water, smoke, and soft drinks are not consistent, and you can separate the parts that make up each. Sodium has a definite and consistent composition, so it is a pure substance.