Which compound would you expect to be hydrated more rapidly?.
Answers
The compound that is expected to be hydrated more rapidly is the one that has greater polarity or is more ionic. When salt is added to water, it dissolves and hydrates.
The ions become solvated or surrounded by water molecules. The greater the polarity, the stronger the attraction between the salt and the water, resulting in faster hydration. In addition, hydration is directly proportional to the surface area of the salt exposed to water. More surface area implies more salt exposure to water, leading to faster hydration.
Therefore, a hydrated salt with a large surface area will dissolve more quickly and is expected to hydrate more rapidly than one with a smaller surface area or less polarity. The most common example of a hydrated salt that dissolves rapidly in water is table salt (NaCl), also known as sodium chloride. Sodium chloride is a salt that contains an ionic bond between sodium and chlorine ions.
Sodium ions are positively charged, while chlorine ions are negatively charged. Water molecules are polar, and the positively charged ions are attracted to the negatively charged end of the molecule. When sodium chloride is added to water, it dissolves quickly and hydrates.
You can learn more about polarity at: brainly.com/question/1946554
#SPJ11
Related Questions
How many atoms are in 18.99g of fluorine?
Answers
18.99g/mol F = 1 mole of F
1 mole is equal to Avogadro's number which is:
\(6.02214076 × {10}^{23} \)
Therefore, one mole of fluorine has 6.02214076 × 10^23 fluorine atoms.
Answer:
Explanation:
The Molar mass of fluorine= 18.99
Moles=mass/molar mass=18.99/18.99=1
Numbers of atoms=1x6.02x10^23=6.02x10^23
I hope it helps
If Sara was planning a wedding and wanted to have a sculpture of a heart made of
butter at the reception, describe how Sara could test the temperature range at
which the heart would remain a solid.
Answers
Answer:
check the Ac so ya so ya so ya so ya so ya so ya
Answer:
Butter stored in a refrigerator is a solid. Sara should put a thermometer in the refrigerator for an hour and record this temperature.
Uranium-235, uranium-238 and uranium-239 are different
A) elements.
B) ions of the same element.
C) isotopes of the same element.
D) none of the above
Answers
Answer:
C
Explanation:
they are of same element but different mass no.
in chemistry language we call them isotopes
A 96,000 gallon pool has a free chlorine level of 1. 4 ppm and a total chlorine level of 1. 8. It takes 2 ounces of dry chlorine (at 67%) to raise a 10,000 gallon pool's chlorine level 1 ppm. How much chlorine is needed to reach break point chlorination? Show all work
Answers
To reach break point chlorination in a 96,000 gallon pool with a difference of 0.4 ppm between the free chlorine and total chlorine levels, approximately 7.68 ounces of chlorine is needed.
To calculate the amount of chlorine needed to reach break point chlorination in a 96,000 gallon pool, we first need to find the difference between the total chlorine and free chlorine levels. Break point chlorination is achieved when the free chlorine level equals the total chlorine level.
Given that the free chlorine level is 1.4 ppm and the total chlorine level is 1.8 ppm, the difference between them is:
1.8 ppm - 1.4 ppm = 0.4 ppm
Now, we need to determine the amount of chlorine required to raise the free chlorine level by 0.4 ppm in a 10,000 gallon pool. The given information states that it takes 2 ounces of dry chlorine (67% concentration) to raise a 10,000 gallon pool's chlorine level by 1 ppm.
To calculate the amount of chlorine required to raise the free chlorine level by 0.4 ppm in a 10,000 gallon pool, we can set up a proportion:
2 ounces / 1 ppm = X ounces / 0.4 ppm
Solving for X (the amount of chlorine needed for 0.4 ppm increase in a 10,000 gallon pool):
X = (2 ounces / 1 ppm) * 0.4 ppm = 0.8 ounces
Now, we can calculate the amount of chlorine needed for the 96,000 gallon pool by scaling the chlorine required for the 10,000 gallon pool:
Amount of chlorine needed = (0.8 ounces / 10,000 gallons) * 96,000 gallons
Amount of chlorine needed = 0.8 ounces * 9.6 = 7.68 ounces
Therefore, approximately 7.68 ounces of chlorine is needed to reach break point chlorination in the 96,000 gallon pool.
For more such question on chlorination. visit :
https://brainly.com/question/24218286
#SPJ8
for the reaction below, what mass of hf must react with excess sio2 to produce 345 kj of energy? sio2 4 hf sif4 2 h2o h rxn = –184 kj a) 42.7 g b) 37.5 g c) 150 g d) 107 g e)
Answers
The correct answer is c) 150 g.
The mass of HF required is approximately 150 g.
How to determine the mass of HF required?To determine the mass of HF required to produce 345 kJ of energy, we need to use the given enthalpy change of the reaction (ΔH = -184 kJ) as well as the stoichiometry of the reaction.
From the balanced chemical equation, we can see that 4 moles of HF produce -184 kJ of energy. We can set up a proportion to calculate the mass of HF required:
(4 moles HF / -184 kJ) = (x moles HF / -345 kJ)
Solving for x, we find:
x = (4 moles HF / -184 kJ) * (-345 kJ)
x ≈ 7.5 moles HF
To convert moles of HF to grams, we use the molar mass of HF (20.01 g/mol):
Mass of HF = 7.5 moles HF * 20.01 g/mol
Mass of HF ≈ 150 g
Therefore, the mass of HF required to produce 345 kJ of energy is approximately 150 g. The correct answer is c) 150 g.
Learn more about mass
brainly.com/question/11954533
#SPJ11
What happens when the bond between the 2nd and 3rd phosphate is broken?
Answers
When the bond between the 2nd and 3rd phosphate of ATP is broken, the energy stored in the bond is released.
What is ATP?Adenosine triphosphate (ATP) is a nucleotide that contains a sugar, ribose, and three phosphate groups in a chain.
The bond that connects the second and third phosphate groups is called a high-energy phosphate bond, and it is the main energy carrier in cells.
When the bond between the 2nd and 3rd phosphate is broken, the energy stored in the bond is released. The bond is broken through a hydrolysis reaction in which water is added to break the bond, releasing energy and producing adenosine diphosphate (ADP) and inorganic phosphate (Pi).
ATP → ADP + Pi + Energy
The energy released from breaking the high-energy bond between the second and third phosphate groups in ATP is used by the cell to drive endergonic (energy-requiring) reactions.
Thus, ATP is often referred to as the energy currency of the cell.
Therefore, when the 2nd and 3rd bond breaks the energy stored in the bond is released.
Learn more about bonds on:
https://brainly.com/question/29282058
#SPJ11
What is the identity of the alkali metal cation: li li , na na , k k , rb rb , or cs cs
Answers
In the long version of the Periodic table, the elements Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), and Francium (Fr) collectively make up one group. Because their oxides and hydroxides are soluble in water and produce a potent alkaline (basic) solution, these metals are collectively referred to as alkali metals.
What do alkali metal's flame colors signify?
Alkali metals' distinctive flame colors—red, yellow, violet, crimson, and blue for Li, Na, K, Rb, and Cs, respectively—are qualitative markers of the contemporary analytical techniques used to calculate the quantities of alkali-metal salts in aqueous solution.
In comparison to other metals, how soft or hard are alkali metals?Most other metals are harder than alkali metals. The most reactive elements in this category are cesium and francium.
Learn more about alkali metals here:-
https://brainly.com/question/27187436
#SPJ4
numerical Through the two ends of glass-tube of lenght 2 meters, hydrogen chloride and ammonia gases are allowed to enter.At what distance ammonium chloride will first appear?
Answers
Answer:
81cm is the answer.
Explanation:
Let X be the length from the HCl end, and therefore, (2-x) from the other end.
For X length, rate of diffusion is :
dx/dt=P A/(Square root of 36.5T
d(2−x)/dt=PA/(square root of 17 T
Dividing both we get;
X/(2−x)= square root of 36.5/17
X=2/2.466=0.81 or81cm
All compounds of carbon are made by _____.
covalent bonding
ionic bonding
metallic bonding
transfer of electrons
Answers
Answer:
Covalent bonding
Explanation:
Covalent bonding is the bond that holds all compound that are formed by carbon.
It is a very strong intermolecular bond that holds the structure of carbon compounds in place.
Principally, covalent compounds are formed by sharing of electrons between contributing atoms.
Covalent bonds are the strongest bond types. A reason for this conclusion can be see in case of diamond. Here, there is a covalent linkage between the atoms present and the strength of these bonds is responsible for how strong the diamond crystal is
Answer:
Covalent Bonding
Explanation:
Got it right on Odessyware!!! ;) Have a nice day!
Which group on the periodic table has the greatest number of metallic elements? O A. Group 18 (VIIIA) O B. Group 2 (IIA) OC. Group 13 (IIIA) O D. Group 7 (VIIB)
Answers
Answer: 18
Explanation:
i took a test and reviewed it and it said 18
On the periodic table, group IA has the most metallic elements.
What is Periodic table?All identified chemical elements are arranged in rows (referred to as periods) and columns (referred to as groups) in the periodic table of chemical elements, also known as the periodic table, in ascending order of atomic number.Groups are the names given to the periodic table's columns. In the table, individuals who belong to the same group make bonds of the same kind and have an equal number of electrons in their atoms' outermost shells. The periods refer to the horizontal rows.Elements in the periodic table can be divided into groups and eras. The periodic table is organized into horizontal rows for periods and vertical columns for groupings. As you advance, the atomic number grows.To learn more about Periodic table refer to:
https://brainly.com/question/15987580
#SPJ2
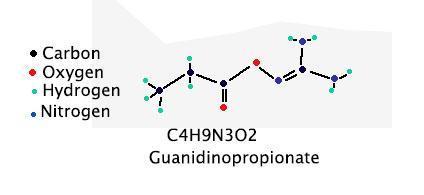
does someone know how to draw a structural formula of H1O2N3C4 please
Answers
Answer:
This could be guanidinopropionate. A drawing of the structure is attached.
Explanation:
There are other possible structures with this formula. The one shown in the attached diagram is one possibility. It is named, unfortunately, guanidinopropionate.
FILL THE BLANK. ibm's watson utilizes a massively parallel, text mining–focused, probabilistic evidence-based computational architecture called ________.
Answers
IBM's Watson utilizes a massively parallel, text mining-focused, probabilistic evidence-based computational architecture called DeepQA.
IBM's Watson utilizes a massively parallel, text mining-focused, probabilistic evidence-based computational architecture called DeepQA.
IBM's Watson utilizes a massively parallel, text mining-focused, probabilistic evidence-based computational architecture called DeepQA.
IBM's Watson utilizes a massively parallel, text mining-focused, probabilistic evidence-based computational architecture called DeepQA.
Learn more about IBM's Watson
brainly.com/question/29459879
#SPJ11
which of the following molecules has the greatest affinity for binding electrons? question 10 options: ubiquinone (q) nadh o2 cytochrome c
Answers
The molecule with the greatest affinity for binding electrons is oxygen (\(O_2\)).
This is because oxygen has a very high electronegativity, meaning it attracts electrons very strongly. When oxygen binds to electrons, it becomes negatively charged, which allows it to form strong bonds with other molecules. This is why oxygen is such an important molecule in cellular respiration, where it accepts electrons from other molecules and ultimately helps produce ATP, the energy currency of cells.
While the other molecules listed (ubiquinone, NADH, and cytochrome c) are also involved in electron transport and have some affinity for binding electrons, none of them have as high an affinity as oxygen. Ubiquinone and cytochrome c both function as electron carriers, but they do not actually bind electrons themselves. NADH is a reducing agent, meaning it donates electrons to other molecules, but it does not have as high an affinity for electrons as oxygen.
Overall, oxygen is the molecule with the greatest affinity for binding electrons, making it a crucial component of many cellular processes.
Learn more about role of oxygen here: https://brainly.com/question/31062386
#SPJ11
The vertical columns on the periodic table are known as:.
Answers
Answer:
groups
Explanation:
In chemistry, the vertical columns are known as groups and the horizontal columns are known as periods.
13. Which statement best describes an element? *
O any combination of two or more atoms of different types
a pure substance made up of only one kind of atom
O
a substance containing only water molecules
O any kind of crystal
Answers
Explanation:
Distinguish chemical substances from mixtures
Key Points
Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated into their original components.
A chemical substance is composed of one type of atom or molecule.
A mixture is composed of different types of atoms or molecules that are not chemically bonded.
A heterogeneous mixture is a mixture of two or more chemical substances where the various components can be visually distinguished.
A homogeneous mixture is a type of mixture in which the composition is uniform and every part of the solution has the same properties.
Various separation techniques exist in order to separate matter, including include distillation, filtration, evaporation and chromatography. Matter can be in the same phase or in two different phases for this separation to take place.
Terms
substanceA form of matter that has constant chemical composition and characteristic properties. It is composed of one type of atom or molecule.
elementA chemical substance that is made up of a particular kind of atom and cannot be broken down or transformed by a chemical reaction.
mixtureSomething that consists of diverse, non-bonded elements or molecules.
Answer:
a pure substance made up of only one kind
Explanation:
Explain why copper is formed at the cathode during the electrolysis of its 2
salts. *
Your answer
T
Answers
the chemist adds m silver nitrate solution to the sample until silver chloride stops forming. he then washes, dries, and weighs the precipitate. he finds he has collected of silver chloride. calculate the concentration of iron(iii) chloride contaminant in the original groundwater sample.
Answers
The concentration of iron(iii) chloride contaminant in the original groundwater sample is (C1 × V1 / V) × 162.2 g/mol.
Given that the chemist adds m silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected of silver chloride. Let us calculate the concentration of iron(iii) chloride contaminant in the original groundwater sample.Calculating the concentration of iron(iii) chloride contaminant in the original groundwater sample
Here is the given information;
Mass of silver chloride precipitate = m grams
Volume of groundwater sample taken = V ml
Volume of AgNO3 solution used = V1 ml
Concentration of AgNO3 solution = C1
Molar Mass of AgCl precipitated = 143.5 g/mol
The molarity of AgNO3 solution is given as;
Molarity of AgNO3 = Number of equivalents / Volume of solution in liters
We know that 1 mole of AgNO3 gives 1 mole of AgCl, i.e., AgNO3 is equivalent to AgCl.Therefore, the number of equivalents of AgNO3 is the same as the number of equivalents of AgCl.
Number of equivalents of AgNO3 = C1 × V1
Number of equivalents of AgCl = m / 143.5 g/mol
Concentration of FeCl3 = (Number of equivalents of FeCl3 / Volume of sample in liters) × Molar mass of FeCl3
Number of equivalents of FeCl3 = Number of equivalents of AgNO3
Number of equivalents of FeCl3 = C1 × V1
Concentration of FeCl3 = (C1 × V1 / V) × Molar mass of FeCl3
Concentration of FeCl3 = (C1 × V1 / V) × 162.2 g/mol
Hence, the concentration of iron(iii) chloride contaminant in the original groundwater sample is (C1 × V1 / V) × 162.2 g/mol.
To know more about contaminant visit:
https://brainly.com/question/28328202
#SPJ11
The combustion of glucose, c6 h12 o6 (s), produces carbon dioxide, co2 (g), and water, h2 o(g), according to the equation below. upper c subscript 6 upper h subscript 12 upper o subscript 6 (s) plus 6 upper o subscript 2 (g) right arrow 6 upper c upper o subscript 2 (g) plus 6 upper h subscript 2 upper o (l). the enthalpy of the reaction is –2,840 kj. what is the heat of combustion, per mole, of glucose?
Answers
The heat of combustion per mol of the glucose is 2840 kJ.
A reaction that involves the burning of a compound in the presence of air or oxygen is called combustion. In the absence of oxygen combustion of a compound cannot take place.
It is given that the combustion of glucose produces carbon dioxide and water. The balanced chemical reaction for the given process is expressed as:
C6H12O6 (s) + 6O2 (g) ------> 6CO2 (g) + 6H2O (l)
The energy released from this reaction is -2840 kJ. The above equation clearly shows that 1 mol of glucose is used during the reaction and the energy produced is -2840 kJ.
Therefore, the heat of combustion per mol of the glucose is 2840 kJ.
Learn more about combustion here:
https://brainly.com/question/13096727
#SPJ4
Consider this reaction:
At a certain temperature it obeys this rate law.
rate
Suppose a vessel containsat a concentration of. Calculate the concentration ofin the vesselseconds later. You may assume no other reaction is important
Answers
The concentration of A after 30 seconds when the given reaction obeys the rate law rate = k[A]²[B].
We use the initial concentration of A and B and the rate constant of the reaction to find the rates at these concentrations. Using the integrated rate law for a second-order reaction, we find the concentration of A after 30 seconds to be 0.0934 M.
Given reaction obeys the rate law, rate=k[A]²[B].
Here, the initial concentration of A= 0.10 M,
initial concentration of B = 0.05 M, and
rate constant, k = 2.0 × 10⁻⁴ M⁻¹s⁻¹
We have to find the concentration of A, after 30 seconds.
To find the concentration of A, we need to know the rate at 0.10 M and 0.05 M. Therefore, we have to calculate the rates at these concentrations.
rate1 = k[A]²[B]
= (2.0 × 10⁻⁴ M⁻¹s⁻¹)(0.10 M)²(0.05 M)
= 1.0 × 10⁻⁷ M/srate2
= k[A]²[B] = (2.0 × 10⁻⁴ M⁻¹s⁻¹)(0.09 M)²(0.04 M)
= 6.48 × 10⁻⁸ M/s
Using the integrated rate law for a second-order reaction: [A] = [A]₀ - kt where [A]₀ = initial concentration of A, k = rate constant, and t = time in seconds.
We know [A]₀ = 0.10 M and k = 2.0 × 10⁻⁴ M⁻¹s⁻¹.
Substituting the values in the above equation, we get: [A] = [A]₀ - kt= 0.10 M - (2.0 × 10⁻⁴ M⁻¹s⁻¹)(30 s)≈ 0.0934 M
Therefore, the concentration of A in the vessel after 30 seconds is 0.0934 M.
This question requires us to calculate the concentration of A after 30 seconds when the given reaction obeys the rate law rate = k[A]²[B].
We are given the initial concentration of A and B and the rate constant of the reaction. To find the concentration of A after 30 seconds, we need to calculate the rates at the initial concentrations of A and B.
Using the integrated rate law for a second-order reaction, we can find the concentration of A at any given time. We substitute the given values in the formula and solve for [A]. We get the concentration of A as 0.0934 M after 30 seconds. This calculation is based on the assumption that no other reaction is important.
The concentration of A after 30 seconds when the given reaction obeys the rate law rate = k[A]²[B]. We use the initial concentration of A and B and the rate constant of the reaction to find the rates at these concentrations. Using the integrated rate law for a second-order reaction, we find the concentration of A after 30 seconds to be 0.0934 M. This calculation assumes that no other reaction is important.
To know more about concentration visit:
brainly.com/question/13872928
#SPJ11
which of the following statements are true? check all that apply. view available hint(s)for part a which of the following statements are true?check all that apply. after 1 hour , less than 50 % of the original atoms in the container will have decayed. after 1 hour , more than 50 % of the original atoms in the container will have decayed. after 2 hours , 50 % of the original atoms in the container will have decayed. after 4 hours , 25 % of the original atoms will have decayed. after 4 hour s, the total number of atoms in the container will be reduced by 75 % .
Answers
The correct answers are: After 1 hour, less than 50% of the original atoms in the container will have decayed, and after 4 hours, 25% of the original atoms will have decayed. The other three statements are not true.
Radioactive decay is a process by which unstable atoms spontaneously emit particles and energy. As atoms decay, the number of unstable atoms decreases, leading to a decrease in the total number of atoms present in a sample over time. For a given sample, the fraction of atoms that remain after a certain amount of time is called the decay rate.
In the given question, after 1 hour, less than 50% of the original atoms in the container will have decayed. This is because the decay rate of the sample will be less than 50%. Similarly, after 4 hours, the total number of atoms in the container will be reduced by 25%. This is because the decay rate of the sample is 1/4 or 25%. Therefore, the total number of atoms in the container will be reduced by 25%.
However, after 1 hour, more than 50% of the original atoms in the container will not have decayed. This is because the decay rate of the sample will be less than 50%. Similarly, after 2 hours, 50% of the original atoms in the container will not have decayed, and after 4 hours, the total number of atoms in the container will not be reduced by 75%. This is because the decay rate of the sample is only 1/4 or 25%. Therefore, the total number of atoms in the container will be reduced by 25%.
For more such questions on atoms
https://brainly.com/question/26952570
#SPJ11
Which statement(s) about electrons is/are TRUE?
SELECT ALL THAT APPLY
a
When they were first discovered, they were named
"corpuscles."
b They have a negative charge.
с
They have a unique charge-to-mass ratio
depending on the element.
d They have a tiny but significant mass.
Answers
Answer:
All are correct
Explanation:
a. when Thomson discovered electrons he called them copuscles.
b. Millikan calculated chage on electron which is -1.6022 x \(10^{-19}\)C
c. Charge to mass ratio of electron is fixed that is 1.758820 x \(10^{11}\)C/kg
d. Although electons have mass (9.1 x \(10^{-31}\)kg) but is very small therefore it is not taken while calculating mass of atom.
Fe(OH)2+HCI--> FeCl2+H2O balanced
Answers
Answer:
Fe(OH)2 + 2HCl ---> FeCl2 + 2H2O
I think
if you insert 2.75 grams of co how many grams of H2 are also used?
Answers
The mass of H₂ used in the reaction, given that 2.75 g of CO was inserted is 0.39 grams
How do i determine the mass of H₂ used?The mass of H₂ used in the reaction can be obtained as illustrated below:
Balanced equation:
CO + 2H₂ -> CH₃OH
Molar mass of CO = 28 g/molMass of CO from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 2 × 2 = 4 gFrom the balanced equation above,
28 grams of CO required 4 grams of H₂
Therefore,
2.75 grams of CO will require = (2.75 grams × 4 grams) / 28 grams = 0.39 grams of H₂
Thus, we can conclude that the mass of H₂ used in the reaction is 0.39 grams
Learn more about mass:
https://brainly.com/question/21940152
#SPJ1
How does an emerging idea differ from scientific consensus? Which best describes emerging scientific ideas?
Answers
Emerging scientific ideas are new theories or ideas that are gaining attention in the scientific community, but have not yet been fully accepted or confirmed.
Emerging ideas refer to the new and innovative ideas or theories that have yet to gain full scientific acceptance. While a scientific consensus is a view or theory that has been universally accepted and confirmed by multiple experiments or research, an emerging scientific idea is a new and unproven theory or idea that is gaining attention in the scientific community. These emerging ideas may also be referred to as scientific hypotheses. In contrast to scientific consensus, emerging scientific ideas have not yet been subjected to rigorous testing and confirmation.
They are generally proposed to explain new observations or experimental results, which have not yet been fully understood or explained by established scientific theories. Emerging scientific ideas can have the potential to challenge the current scientific consensus. If an emerging scientific idea is found to be valid, it can ultimately lead to the establishment of a new scientific consensus. For example, the emerging scientific idea of the Higgs boson particle led to the discovery of a new field in particle physics, which is now an established scientific consensus.
for such more questions on scientific
https://brainly.com/question/29886197
#SPJ8
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Why is it possible to overdose on Vitamin
A, but not on Vitamin C?
Answers
Answer:
Vitamin overdose occurs when a person ingests far more than the daily recommendation, for an extended period of time. Although the body can excrete excessive amounts of water-soluble vitamins such as vitamin C, it can retain fat-soluble vitamins such as vitamin A, which can be toxic.
what is the IUPAC name to this structure?

Answers
I think this may be hexan-2,3-diol
Cation____ electrons causing the ion to have a ______ charge and anion ______ electrons to have a ________ charge
Answers
Answer:
are electrons causing the ion to have a positive charge and anions are electrons that have a negative charge
Explanation:
when 3.18 g of copper (||) oxide were carefully heated in a stream of dry hydrogen, 2.54 g of copper and 0.72 g of water were formed determine the number of moles of hydrogen atoms which combine with one mole of oxygen atoms
Answers
Answer:
the number of moles of atom is 0.91584
which of the following statements below correctly describes the mechanism involving the acid-catalyzed hydration of alkene with water? group of answer choices the addition of the nucleophile is a fast step. all of the possible choices a carbocation is formed as an intermediate. the addition of the electrophile is a slow step. water removes a proton from the protonated alcohol.
Answers
The mechanism involving the acid-catalyzed hydration of an alkene with water involves the formation of a carbocation intermediate, and the addition of the electrophile is the slow step.
The acid-catalyzed hydration of an alkene with water is a reaction in which an alkene molecule reacts with water in the presence of an acid catalyst to form an alcohol. The mechanism for this reaction involves several steps.
Firstly, the acid catalyst protonates the alkene, forming a carbocation intermediate. This step is usually fast because the alkene acts as a nucleophile and readily accepts a proton from the acid.
Next, water acts as a nucleophile and adds to the carbocation, forming a protonated alcohol. This addition of the nucleophile is typically fast.
Finally, a deprotonation step occurs, where water removes a proton from the protonated alcohol, resulting in the formation of the alcohol product.
Learn more about deprotonation here:
https://brainly.com/question/30706409
#SPJ11