which compound is more soluble in an acidic solution than in a neutral solution? a. pbbr2 chem 1212k spring 2022 dr. stroeva b. cucl2 c. agi d. baf2
Answers
AgI. this is that AgI is a sparingly soluble salt, meaning it has low solubility in water. However, in an acidic solution, the solubility of AgI increases because the acidic environment protonates the iodide ion, forming HI.
The increased concentration of H+ ions shifts the equilibrium towards the dissociation of AgI, resulting in higher solubility. In contrast, PbBr2, CuCl2, and BaF2 do not exhibit a significant change in solubility in acidic versus neutral solutions.
Which compound is more soluble in an acidic solution than in a neutral solution? The given options are a) PbBr2, b) CuCl2, c) AgI, and d) BaF2.
BaF2 is more soluble in an acidic solution than in a neutral solution because, in an acidic solution, the fluoride ions (F-) from BaF2 can react with the excess H+ ions to form HF (hydrofluoric acid). This reaction removes F- ions from the solution, leading to an increased solubility of BaF2 according to Le Chatelier's principle. The other compounds (PbBr2, CuCl2, and AgI) do not exhibit similar behavior in acidic solutions.
To know more about environment protonates Visit;
https://brainly.com/question/31060237
#SPJ11
Related Questions
identify the list in which the atoms are ordered by increasing zeff for a valence electron calculated by the simple or approximate method?
Answers
Atoms in increasing order of Z\(_eff\) for valence electron by simple or approximate method are Si<P<S<Cl.
What is an atom?An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
The protons are positively charged and neutrons are neutral and hence the nucleus is positively charged. The electrons which revolve around the nucleus are negatively charged and hence the atom as a whole is neutral and stable due to presence of oppositely charged particles.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ1
Your question is incomplete, but most probably your full question was,identify the list in which the atoms are ordered by increasing Zeff for a valence electron calculated by the simple or approximate method? A) P<S<Si<Cl B) Si<P<S<Cl C) Ck<S<P<Si D) S<Cl<P<Si E) Si<P<Ck<s
what is the volume occupied by 4 grams of oxygen at room temperature
Answers
Answer:
volume of O2 = 3000cm³
Explanation:
no. of moles= mass of oxygen (g)/molar mass of oxygen(g/mol)
= 4/32
=0.125
volume of oxygen= no. of moles × 24000 cm³ (or 24dm³)
= 0.125 × 24000 cm³
=3000cm³ (or 3dm³)
pls help me with this
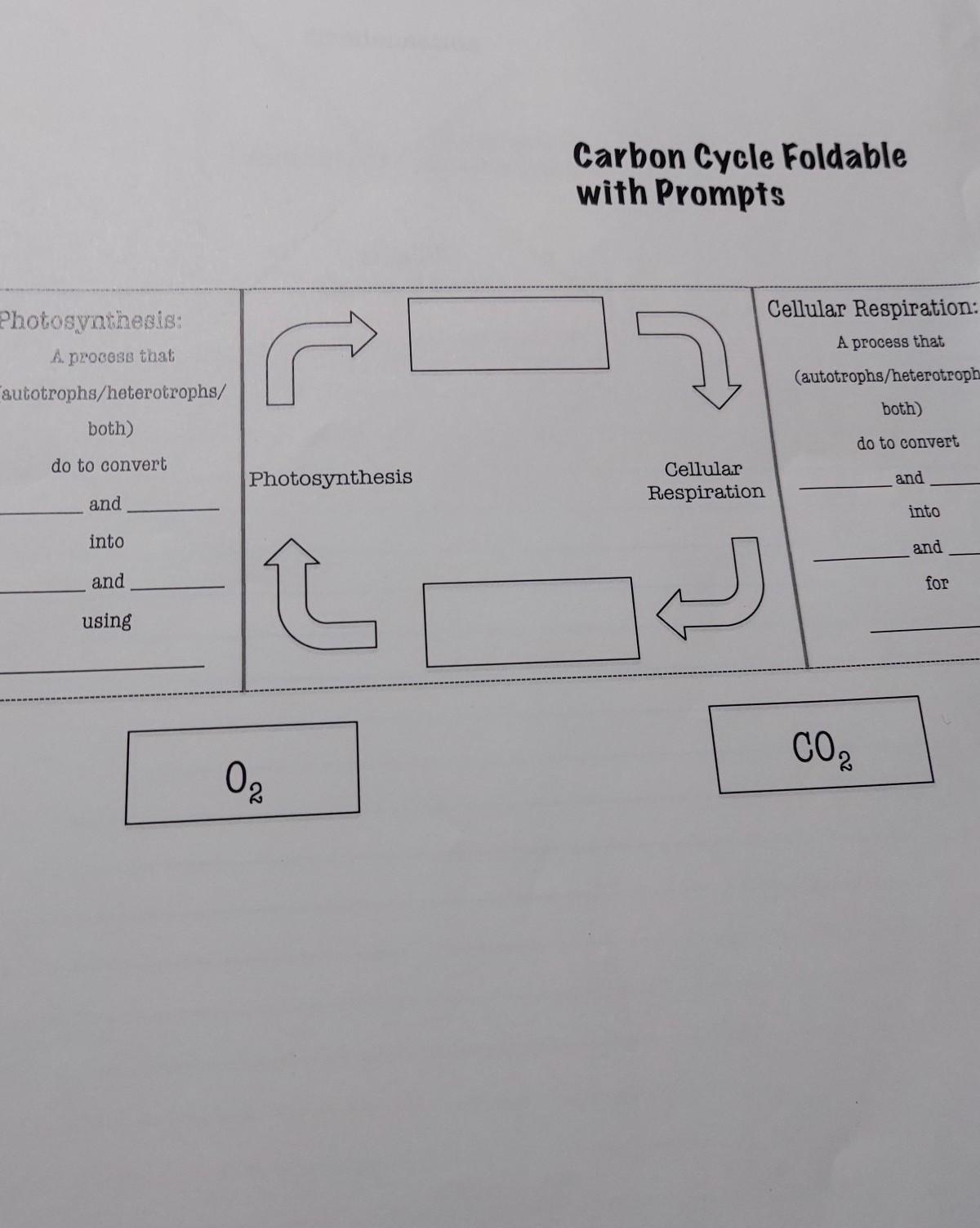
Answers
When gaseous Na+ and Cl− ions form gaseous NaCl ion pairs, 548 kJ/mol
of energy is released. Why, then, does NaCl occur as a solid under ordinary conditions?
Answers
When the gaseous Na+ and Cl− ions form gaseous NaCl ion pairs, 548 kJ/mol of energy is released. This is known that the energy released by combination of oppositely charged ion is called as Lattice energy.
Lattice energy is defined as the energy change upon formation of one mole of a crystalline ionic compound from its constituent ions which are assumed to initially be in the gaseous state. This energy is a measure of the cohesive forces that bind ionic solids. The lattice energy releases even more energy when gas is converted into solid. The ionic bonds are very strong due to high attractive forces holding the positively and negatively charged ions alternatively together. They are packed tightly and the crystal lattice structure is extremely hard to break. Thus at normal room temperature, Sodium chloride occur as solid.
To learn more about Lattice energy please visit:
https://brainly.com/question/4792664
#SPJ4
Which of the following characteristics are true about a typical peptide (amide) bond?
1) The bond is planar.
2) There is free rotation about the carbonyl carbon and nitrogen bond.
3) There is substantial double-bond character to this bond.
4) There is a net negative charge on nitrogen and net positive charge on oxygen.
O Only statements 1, 2, and 4 are correct.
O Only statements 1 and 3 are correct.
O Only statements 2 and 3 are correct.
O All of the listed statements are correct.
Answers
The correct answer is:Only statements 1 and 2 are correct.
1) The bond is planar.
2) There is free rotation about the carbonyl carbon and nitrogen bond.
The bond is planar: True, Peptide bond is a planar bond because it is composed of a double bond between the carbonyl carbon and nitrogen, and the atoms on either side of the double bond are sp2 hybridized. There is free rotation about the carbonyl carbon and nitrogen bond: True, the peptide bond between amino acids in a protein is a single bond, which allows for rotation about the bond, this is one reason proteins can adopt many different conformations.There is substantial double-bond character to this bond: False, the peptide bond is actually a single bond, although it has some double-bond character, it is not a double bond.There is a net negative charge on nitrogen and net positive charge on oxygen: False, both nitrogen and oxygen atoms in a peptide bond are neutral, as there is no charge separation in the bond.
To learn more about typical peptide here:
https://brainly.com/question/15578006
#SPJ4
which protein is sensitive to ca2 and thereby helps initiate contraction?
Answers
The protein that is sensitive to Ca₂ and helps initiate muscle contraction is called troponin.
TroponinsTroponins are a group of proteins that play a crucial role in muscle contraction, particularly in skeletal and cardiac muscles. They are composed of three subunits: troponin C (TnC), troponin I (TnI), and troponin T (TnT).
Troponin C binds to calcium ions (Ca²⁺) and undergoes a conformational change when Ca²⁺ levels increase. This change allows troponin I to release its inhibition on the actin-myosin interaction, allowing muscle contraction to occur. Troponin T anchors the troponin complex to the tropomyosin strands, which are filamentous proteins that regulate access to the myosin-binding sites on actin.
The interaction between troponin and calcium is a crucial step in the excitation-contraction coupling of muscles. When a nerve impulse stimulates muscle contraction, calcium ions are released from the sarcoplasmic reticulum into the muscle cell. The increased calcium concentration binds to troponin C, leading to the removal of tropomyosin from the myosin-binding sites on actin, enabling the myosin heads to attach and generate force, resulting in muscle contraction.
Troponins serve as regulatory proteins, allowing precise control of muscle contraction and relaxation. By responding to changes in calcium levels, they enable the initiation and regulation of muscle contraction in response to neural signals.
learn more about muscle contraction
https://brainly.com/question/25778330
#SPJ11
indicate whether the following reaction involves c–o or o–h bond cleavage of the alcohol molecule.
Answers
The following reaction involves (1) C–O bond cleavage of the alcohol molecule.
When extreme circumstances and an abundance of hydrogen halides are present, the C-O bond in ethers can be broken. Two molecules of alkyl halide are produced as a byproduct of the process involving dialkyl ether. Because of the increased strength of the aryl-oxygen bond, the alkyl-oxygen bond in alkyl aryl ethers is the point of cleavage. The reaction results in the production of phenol and alkyl halide.
As we can see in the given mechanism, after the first step, an intermediate C-O bond is broken by the Br⁻ ion in alcohol, which is an incoming ion via the SN2 mechanism. At this stage, the O-H bond is not broken; only the C-O bond is broken.
The complete question is attached.
You can also learn about SN2 mechanism from the following question:
https://brainly.com/question/7213902
#SPJ4

How do particles that make up the solid, liquid and gas phases differ in terms of distance between particles, kinetic energy and potential energy? ALSO: [2] Explain how the evaporation of water or other liquids from the skin can have a cooling effect. ALSO: [3] Define: vapor pressure, vaporization point and normal vaporization point.
Answers
In terms of distance between particles:
In a solid, particles are tightly packed and have a fixed position and liquid, particles are close to each other but have more freedom of movement but gas, particles are far apart and have complete freedom of movement.
in terms of Kinetic energy solid particles have the lowest kinetic energy liquid, particles have higher kinetic energy compared to a solid and gas particles have the highest kinetic energy.
In terms of Potential energy solid particles have the lowest potential energy liquid particles have a slightly higher potential energy and gas, particles have the highest potential energy.
How do we define?a) Vapor pressure is the pressure exerted by the vapor molecules of a substance when it is in equilibrium with its liquid or solid phase at a given temperature.
b) Vaporization point is the temperature at which a substance changes from a liquid to a gas phase through the process of vaporization.
c) Normal vaporization point is described as the temperature at which a substance changes from a liquid to a gas phase at a standard pressure of 1 atmosphere.
When water or any liquid evaporates from the skin, it absorbs heat energy from the surrounding area, including the skin itself which the absorption of heat energy is due to the higher kinetic energy of the liquid particles near the surface.
Learn more about Vapor pressure at:
https://brainly.com/question/2693029
#SPJ1
Select 3 that apply
What are the equations for the chemical reactions that took place when the acids reacted with active metals?
Zn(s) + 2HCl(aq) → ZnH2 (aq)+ Cl2↑
Mg(s) + 2HCl(aq) → MgH2 (aq)+ Cl2↑
Cu(s) + HCl → nothing happened
Mg(s) + 2HCl(aq) → MgCl2 (aq)+ H2↑
Cu(s) + HCl(aq) → CuCl (aq)+ H2↑
Zn(s) + 2HCl(aq) → ZnCl2 (aq)+ H2↑
Answers
The reaction that take place when active metals react with acids are:
Mg (s) + 2HCl (aq) → MgCl₂ (aq) + H₂ (g)Zn (s) + 2 HCl (aq) → ZnCl₂ (aq) + H₂ (g)What are active metals?Active metals are those metals which are very reactive and are found high above hydrogen in the activity series of metals.
The reactivity of metals is determined by the ability of the meta to give up electrons to form positive ions called cations.
Therefore, active metals are those metals that readily give up their electrons to form cations.
The active metals are found in the group 1A and 2A and 3A of the periodic table.
The active metals include; sodium, potassium, calcium, aluminum, magnesium, zinc etc.
The active metals react with dilute acids to displace hydrogen, resulting in the evolution of hydrogen gas.
Considering the given equations, the reactions of the active metals are:
Mg (s) + 2HCl (aq) → MgCl₂ (aq) + H₂ (g)
Zn (s) + 2 HCl (aq) → ZnCl₂ (aq) + H₂ (g)
Learn more about active metals at: https://brainly.com/question/11889607
#SPJ1
The ____number can vary among atoms of the same element.
Answers
Answer:
Mass number
Explanation:
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons, which would cause a different mass number
Answer:
The answer is mass number
Which best explains how convection affects the atmosphere
Answers
Answer:
The heating of the Earth's surface and atmosphere by the sun drives convection within the atmosphere and ocean. This convection produces winds and ocean currents. The greater the pressure differences between a low-pressure area and a high-pressure area, the stronger the winds
When alkaline hydrolysis was first invented what jobs were people hiring to do?
Answers
When alkaline hydrolysis was first invented, people were hired for various roles related to the process and implementation of this technology. Some of the jobs that emerged include Chemical engineers, Technicians and operators, Waste management specialists, Scientists and researchers.
Chemical engineers: These professionals played a crucial role in developing and optimizing the alkaline hydrolysis process. They were responsible for designing the equipment, developing the necessary chemical reactions, and ensuring the efficient operation of the system.
Technicians and operators: Skilled technicians and operators were hired to operate and maintain the alkaline hydrolysis equipment. They were trained to monitor the process parameters, handle the chemicals involved, and ensure the proper functioning of the system.
Waste management specialists: With the introduction of alkaline hydrolysis as a method for disposal of organic waste, specialized professionals in waste management were employed to oversee the proper handling and treatment of the waste materials. They were responsible for implementing safety protocols, managing waste streams, and complying with environmental regulations.
Scientists and researchers: Alkaline hydrolysis required scientific expertise for continuous improvement and innovation. Scientists and researchers were hired to study the process, analyze the results, and explore potential applications in various fields such as biofuel production and chemical synthesis.
Overall, the introduction of alkaline hydrolysis created employment opportunities for professionals in engineering, chemistry, waste management, and research, among others, as this technology gained recognition and adoption.
To know more about hydrolysis , click here, https://brainly.com/question/31132313
#SPJ11
calculate the volume the gas will occupy if the pressure is increased to 1.89 atm while the temperature is held constant.
Answers
By raising the pressure to 1.89 atm and keeping the temperature constant, 2.86 L volume of gas was consumed.
The initial pressure is 758 torr, and the question specifies a temperature of 21. The initial volume is 5.42 L. 1.89 atm has been given as the final pressure.
Atm units have been used to convert the original pressure.
1 atm Equals 760 torr so,
0.997 atm = 758 torr
In line with Boyle's law: \(P1*V1=P2*V2\)
Where, P1 and V1 stand for, respectively, the starting pressure and volume. The final pressure and volume are P2 and V2, respectively.
so,
0.997 atm * 5.42 L = 1.89 atm * Final volume
Final volume: 2.86 L
Learn more about Boyle's Law:
brainly.com/question/1437490
#SPJ4
The life cycle of silkworm includes ------------- stage after the larvae stage
Answers
Answer:
Pupa Stage
Explanation:
Silk worm consists of four stages- the adult, the egg, the larva (caterpillar) and the pupa stage.
Solve The Combined Gas Law Problem
P₁ = 720 torr
V₁ = 256 mL
T₁= 25.0 °C
P2 = ? torr
V₂ = 250 mL
T2 = 50.0 °C
Answers
Answer:
200 torr
Explanation:
because it have 250ml when we subtract the 50 we get answer
A crude nonacidic product mixture dissolved in diethyl ether contains acetic acid. Describe an extraction procedure that could be used to remove the acetic acid from the ether.
Answers
This problem is describing a mixture composed by diethyl ether and actic acid, in which it is desired to extract the latter somehow.
In this case, it turns out relevant to recall the concept of polarity, hydrogen bonds, and, in general, intermolecular forces. Acetic acid is a highly polar compound, able to have both dipole and hydrogen-bonding interactions, which are by far stronger than nonpolar-based London dispersion forces.
In such a way, since diethyl ether and actic acid are able to interact via dipole-dipole interactions, they latter is able to be dissolved in the former; however, when it is desired to extract the acid, it would be necessary to add a substance able to form H-bonds with acetic acid, which are stronger than the dipole interactions.
Therefore, the best solvent for this extraction will be water due to its higher polarity and superior capacity to form H-bonds.
Learn more:
https://brainly.com/question/3464712https://brainly.com/question/10904296I'm very confused please help

Answers
Answer:
the food chain you mean
Explanation:
pollution than mosquitoes then alligators
a hydrogen tank has a pressure of 101,325 Pa at 30 degrees celcius.At what temperature would its pressure be equal to 1.75 atm?
Answers
The temperature at which the pressure will be equal to 1.75 atm, given that the tank has an initial pressure of 101325 Pa is 257.25 degrees celsius
How do i determine the temperature?First, we shall list out the given parameters from the question. This is shown below:
Initial pressure (P₁) = 101325 Pa = 101325 / 101325 = 1 atm Initial temperature (T₁) = 30 degrees Celsius = 30 + 273 = 303 KFinal pressure (P₂) = 1.75 atmFinal temperature (T₂) =?The final temperature can be obtain as follow:
P₁ / T₁ = P₂ / T₂
1 / 303 = 1.75 / T₂
Cross multiply
1 × T₂ = 303 × 1.75
T₂ = 530.25 K
Subtract 273 to obtain answer in degree celsius
T₂ = 530.25 – 273 K
T₂ = 257.25 degrees celsius
Thus, the temperature required is 257.25 degrees celsius
Learn more about temperature:
https://brainly.com/question/13628594
#SPJ1
> Ha
19. You have found a mysterious substance that appears to be radioactive. You take a sample to a nuclear chemist who
measures out 100 g. of the substance. Thirty hours later, the chemist tests the sample and finds that 6.25 grams of the
sample remains radioactive. What is the half-life of the sample?
> Nu
> Nu
Answers
Answer:
7.5 hours
Explanation:
half life is the a period of time the sample takes to be half the amount it started the period with
it means we can express it as
original amount / (2^n) = current amount
where n is the number of periods
in the problem if we put 100 / (2^n) = 6.25
we will find that n = 4
we divide 30 over 4 and we get that half life of each period will be equal to 7.5
why is the non-luminous flame preferred for heating?
Answers
Answer:
Because it is the hottest zone
Answer:
the non luminous flames are very hot. little light is produced by non luminous flames.
Calculate the osmolarity of a solution that contains 200mM NaCl and 100mM Urea.
Describe what happens to a human cell placed in this solution.
What is the final osmolarity of the: cell solution What direction will water flow?
Answers
Osmolarity of solution that contains 200 mM NaCl and 100 mM Urea: The osmolarity is defined as the total solute concentration per liter of the solution.
To find the osmolarity of a solution that contains 200mM NaCl and 100mM Urea, we have to calculate the total solute concentration first.
The molecular mass of NaCl and Urea are 58.5 g/mol and 60 g/mol respectively.
So the total mass of NaCl and Urea is
(200mM * 58.5 g/mol) + (100mM * 60 g/mol) = 11.7 g/L + 6 g/L
= 17.7 g/L
Now we have to convert grams into moles to calculate the total solute concentration.
The total moles of NaCl and Urea are
(11.7 g/L)/(58.5 g/mol) + (6 g/L)/(60 g/mol) = 0.2 M + 0.1 M
= 0.3 M
Osmolarity = 0.3 Osm/L
Human cell placed in this solution: If we place a human cell in this solution, it will either shrink or swell depending on the tonicity of the solution.
Tonicity is a measure of the effective osmotic pressure gradient between two solutions separated by a semipermeable membrane.
If the concentration of solutes is greater outside the cell, the solution is hypertonic and the cell will lose water. In this case, the cell will shrink.
If the concentration of solutes is greater inside the cell, the solution is hypotonic and the cell will gain water.
In this case, the cell will swell. Final osmolarity of cell solution:
The osmolarity of a cell solution depends on the solutes and water present in it.
If we assume that the cell is isotonic to the solution outside, then the osmolarity of the cell solution will be the same as the solution outside, i.e. 0.3 Osm/L.
Direction of water flow: If the cell is placed in a hypertonic solution, water will flow out of the cell to the outside solution.
If the cell is placed in a hypotonic solution, water will flow into the cell from the outside solution.
If the cell is placed in an isotonic solution, there will be no net flow of water.
To know more about human cell, visit:
https://brainly.com/question/13550579
#SPJ11
IWhich of the following solutions would be most likely to have the highest water concentration?
Multiple Choice hypertonic solution isotonic solution hypotonic solution water concentration and tonicity of a solution cannot be compared
Answers
"Hypotonic solution," refers to a solution with the highest water concentration due to its lower solute concentration compared to the other options, leading to water influx into cells.
A hypotonic solution would be most likely to have the highest water concentration. In a hypotonic solution, the solute concentration is lower than that inside the cell or compared to another solution. As a result, water tends to move into the cell or the solution to equalize the concentration.
When a cell is placed in a hypotonic solution, water molecules will move into the cell through the process of osmosis. This influx of water increases the water concentration inside the cell, leading to cell swelling or even bursting in extreme cases.
Compared to hypertonic and isotonic solutions, a hypotonic solution has a lower solute concentration, allowing for a higher concentration of water molecules. This results in a higher water concentration in the solution. It's important to note that the concept of tonicity is related to the relative solute concentrations between two solutions and their effect on cell osmosis. In this case, a hypotonic solution is characterized by a higher water concentration compared to the other options.
learn more about Hypotonic solution here:
https://brainly.com/question/28020628
#SPJ11
PLEASE ANSWER THIS QUICK 50 POINTS :) RIGHT ANSWERS ONLY

Answers
Following the formula they gave you need to do
i × kb × mass
1 × 2.65 × 2
= 5.3
Change in temperature should be 5.3°C
Make a science problem for me that includes, ecology, circuits, microscopes or astrology that can be solved using an experiment.
Answers
What is the total volume of gaseous products formed when 119 liters of carbon monoxide react completely according to the following reaction? (All gases are at the same temperature and pressure.) carbon monoxide (g) + water(l>carbon dioxide (g) + hydrogen(g) _________ liters products
Answers
To find the volume of gaseous products formed in the given reaction, we have to balance the chemical equation and then use the balanced chemical equation to solve the question.
The balanced chemical equation of the given reaction is as follows:
CO (g) + H2O (l) → CO2 (g) + H2 (g)
Now, we need to determine the mole ratios of reactants and products:
1 mole of CO reacts with 1 mole of H2O to produce 1 mole of CO2 and 1 mole of H2.
As given, we have 119 liters of CO, we need to calculate the amount of H2O required to react with 119 liters of CO:
1 mole of CO occupies 22.4 L at STP.
Therefore, 119 L of CO contains 119/22.4 = 5.31 moles of CO.
From the balanced chemical equation, we can see that 1 mole of CO reacts with 1 mole of H2O.
Therefore, 5.31 moles of CO will react with 5.31 moles of H2O.
So, the volume of H2O required to react with 119 L of CO can be calculated as follows:
5.31 moles of H2O occupy 5.31 × 18 L = 95.58 L
Now, we have the volume of H2O required to react with 119 L of CO.
We know that, at the same temperature and pressure, the volume of the gaseous product is directly proportional to the number of moles of the gaseous product.
So, the volume of gaseous products formed in the given reaction can be calculated as follows:
1 mole of CO2 occupies 22.4 L at STP.
Therefore, 5.31 moles of CO2 will occupy 5.31 × 22.4 L = 119.2 L
1 mole of H2 occupies 22.4 L at STP.
Therefore, 5.31 moles of H2 will occupy 5.31 × 22.4 L = 119.2 L
Therefore, the total volume of gaseous products formed in the given reaction is 119.2 L. Answer: 119.2
to know more about chemical balance refer here:
https://brainly.com/question/28294176#
#SPJ11
D. When the astronauts get this water in space they perform electrolysis and only are able to
experimentally make 43,200g of O₂. Using this as your experimental (actual) yield and your answer
from part C as your theoretical, calculate the percent yield of Oxygen.
actual yield
theoretical yield
x 100%
percent yield
=
Answers
Answer:
The theoretical yield of oxygen (O2) can be calculated using the balanced chemical equation:
2 H2O(l) → 2 H2(g) + O2(g)
From part (c), we calculated that 90.0 g of water (H2O) can produce 31.98 g of oxygen (O2). Therefore, the theoretical yield of oxygen from 43,200 g of water is:
theoretical yield = (31.98 g O2 / 90.0 g H2O) x 43,200 g H2O
theoretical yield = 15,379.2 g O2
The percent yield of oxygen can be calculated using the formula:
percent yield = (actual yield / theoretical yield) x 100%
Substituting the given values, we get:
percent yield = (43,200 g / 15,379.2 g) x 100%
percent yield ≈ 280.9%
This result seems unusually high, and suggests an error in the calculations or experimental data. A percent yield greater than 100% indicates that the actual yield is greater than the theoretical yield, which is usually not possible due to limitations in the reaction conditions or experimental procedures.
What is an ecosystem. A.It is a single plant and a single animal that live in given area B. it is a community of plants, animals and their physical surrounding. C it is the members of the same species in an area. D it is one species that lives in a given area GIVE BRANLIYST
Answers
Answer:
B. it is a community of plants,animals and there physical surrounding.
298 g of BaSO4
express your answer using three significant figures
Answers
298 g of BaSO4 is the mass of Barium Sulfate which is expressed using three significant figures.
Explain about Barium Sulfate:Significant figures are the digits in a measurement that are known with certainty, plus one last digit that is uncertain. In this case, the number 298 is made up of three significant figures, which are 2, 9, and 8.The use of significant figures helps to communicate the level of precision or uncertainty in a measurement. In scientific or technical work, it is important to use the correct number of significant figures in order to indicate the precision of a measurement and to avoid errors in calculations.When working with measurements, it is important to be consistent with the number of significant figures used throughout your calculations. When you perform mathematical operations on measurements, the result should have the same number of significant figures as the measurement with the least significant figures.In this case, 298 g of BaSO4 is a precise measurement, with three significant figures. It is important to keep this same number of significant figures in any further calculation that involves it.To learn more about significant figures refer to:
https://brainly.com/question/24491627
#SPJ1
2 AgNO3(aq) + K2SO4(aq) → 2 KNO3(aq) + Ag2SO4(s) The net ionic reaction for the balanced equation shown above is? a. K+ + NO 3 - KNO3 b. 2K+ + 30% - K2SO4. c. 2Ag+ + so - Ag2SO4. d. H+ + OH -- H2O.
Answers
Option C. 2Ag+ + SO42- --> Ag2SO4 is the The net ionic reaction for the balanced equation.
Mass and charge must be equal in net ionic equations. By balancing by mass, one may make sure that each element is present in equal amounts on both the product and reactant sides. Making ensuring that the total charge is the same on both sides of the equation is known as balancing by charge. A chemical equation for a process known as the net ionic equation only includes the species that are really involved in the reaction. In double displacement reactions, redox reactions, and acid-base neutralization processes, the net ionic equation is frequently employed.
The complete question is :
The net ionic reaction the balanced equation shown above is
2AgNO3 (aq) +K2SO4 (aq) --> 2KNO3 (aq)+ Ag2SO4 (s)
A. Ag+ + NO3- --> AgNO3
B. H+ + OH- --> H2O
C. 2Ag+ + SO42- --> Ag2SO4
D. 2K+ +SO4(2-) -->K2SO4
E.K+ +NO3- -->KNO3
learn more about net ionic equation here:
https://brainly.com/question/13879496
#SPJ4
Jackie performs an experiment on how the type of music played in a room can affect
plant growth. In her experiment, the independent variable is the—
A. Color of flower
B. Type of music
C. Height of plant
D. Type of plant
Answers
Answer:
B. Type of music.
Explanation:
"how the type of music played in a room can affect plant growth."
The independent variable is the variable that is changed for the purpose of the experiment. The type of music is the independent variable here.