Which bonds are stronger: the bonds formed or the bonds broken?
Answers
The strength of bonds formed and broken depends on the specific chemical reaction involved. In some reactions, the bonds formed are stronger than the bonds broken, while in other reactions, the opposite is true.
When a chemical reaction is exothermic, meaning that it releases energy, the bonds formed are typically stronger than the bonds broken. This is because energy is released when the bonds are formed, indicating that they are more stable and stronger than the bonds that were broken.
On the other hand, in an endothermic reaction, meaning that it absorbs energy, the bonds broken are usually stronger than the bonds formed. This is because energy is required to break the existing bonds, indicating that they are stronger and more stable than the new bonds that are formed.
To know more about chemical reaction, refer here:
https://brainly.com/question/29762834#
#SPJ11
Related Questions
Justify that electrochemical methods depends upon electrochemical cells?
Answers
For conducting chemical reactions, electrochemical methods rely on electrochemical cells to supply the necessary voltage and current.
How are electrochemical cells dependent on them?
Electrochemical cells, which are machines that use electricity to move ions from one electrode to another, are the foundation of electrochemical methods. I
In an electrochemical cell, a voltage source provides the energy required to move the ions while also driving their motion. A chemical reaction between the electrodes and the ions is sparked by this energy, and the result is a current.
The concentration of the electroactive species in the solution is then determined using the current generated. As a result, electrochemical cells are necessary for electrochemical methods. Chemical reactions can also be used to create energy in electrochemical cells.
To learn more about electrochemical cells refer :
https://brainly.com/question/10470515
#SPJ1
When working with scientific data, the average of a data set is called the
Answers
Answer:
Its called the mean of the data.
Explanation:
Balance the nuclear equation by giving the mass number, atomic number, and element symbol for the missing species.
¹⁰B + ⁴He → ____
Answers
Balanced nuclear equation by giving the mass number, atomic number, and element symbol for the missing species is ¹⁰B + ⁴He → ¹⁴N + 1¹H
The missing species in the given nuclear equation is ¹⁴N and 1¹H.
Let's write the mass number, atomic number, and element symbol for the missing species:
Mass number of ¹⁴N = 14
Atomic number of ¹⁴N = 7
Element symbol of ¹⁴N = N (since the atomic number of nitrogen is 7)
Mass number of 1¹H = 1Atomic number of 1¹H = 1
Element symbol of 1¹H = H (since the atomic number of hydrogen is 1)Therefore, the main answer of the balanced nuclear equation is: ¹⁰B + ⁴He → ¹⁴N + 1¹H.
In order to balance a nuclear equation, it is essential that the total mass number and the total atomic number is conserved.
Balancing nuclear equations requires both the knowledge of atomic structure as well as knowledge of the laws of conservation of mass and charge.
A balanced nuclear equation is essential in understanding nuclear reactions and in predicting the outcomes of nuclear reactions.The balanced nuclear equation for the given nuclear equation is:¹⁰B + ⁴He → ¹⁴N + 1¹H. Conclusion:Thus, the missing species in the given nuclear equation is ¹⁴N and 1¹H.
For more information on nuclear equation kindly visit to
https://brainly.com/question/32262848
#SPJ11
How many moles of water are in 1.23x10^18 water molecules?
in scientific notation
Answers
Answer:
1.23x10^24 atoms/6.022x10^23 atom/mol = 2.04 mol H20
Explanation:
What does it mean if you have a metallic taste in your mouth.
Answers
How many significant figures should be reported in the answer to the following calculation?
(43.980) × (19.0023 + 25) =
Answers
explanation: took that test
I need answers now!
Which statement describes the focus of an earthquake?
O It creates stress in rock.
O It develops in the lithosphere.
Olt lies above the surface where rock breaks.
O. It begins about 5 kilometers below Earth's surface.
Answers
Answer:
B It develops in the lithosphere
Explanation:
Answer:
B
Explanation:
I did the quiz
Structure of -a-D-maltose. Can someone help?
Answers
Answer:
-a-D-Maltose is a disaccharide composed of two glucose molecules linked together by a glycosidic bond. The two glucose molecules are linked together in an alpha-1,4-glycosidic bond, meaning that the anomeric carbon of the first glucose molecule is linked to the fourth carbon of the second glucose molecule. The two glucose molecules are also in the D-configuration, meaning that the hydroxyl group on the anomeric carbon of the first glucose molecule is on the right side when viewed from the bottom of the molecule.
Explanation:
please mark me brainliest
Given: K for acetic acid is 1.8 X 10–5You are titrating 0.108 M NaOH into 142.0 ml of acetic acid of unknown concentration. You have an indicator that will change color when equivalence is reached. At equivalence, you have added 72.0 ml of the base. Calculate molarity of the acid. What is the pH of the solution at the equivalence point? Now that you know the molarity of the acid, find pH when you mix 50.0ml of the acid with 75.0 ml of the same NaOH solution. Now you are working with different acid and base, both weak. K for the acid is 2.25 X 10-5. You mix 63 ml of 0.275 M acid with 55.0 ml of a weak base of concentration 0.188 M. Find pH
Answers
Answer:
Explanation:0.493 M NaOH means 0.493 mol NaOH/L
mols
mols = ------ x L
L
mols = M x V
In a titration procedure, 40.57 mL of 0.493 M NaOH solution was used. How many mols NaOH did this volume of NaOH solution contain?
mols = M x V
0.493 mols NaOH
mols = ----------------------- x 0.04057 L
L
mols = 0.0200 mols NaOH
Which is true and explain?
a. An increased number of protons and electrons increases the attraction between them.
b. An increased number of protons and electrons decreases the attraction between them.
C. An increased number of protons and electrons has no effect on the attraction between them
Answers
Answer:
a. An increased number of protons and electrons increases the attraction between them.
Explanation:
The positively charged protons in the nucleus attract the negatively charged electrons. As the number of protons in the nucleus increases, the electronegativity or attraction will increase.
The electron configuration of an element is 1s22s22p6. Describe what most likely happens when an atom of this element comes near an atom having seven valence electrons.
Answers
Answer: Nothing will happen
Explanation:
Elements want to fill their valence shell with electrons (8).
Referring to both of the table below we will know that the element with that electron configuration is Neon (Ne). It is on the far right side of the periodic table so it contains all 8 electrons in its valence shell. The elements on the right side of the periodic table are called noble gases and are not reactive because they have the maximum number of electrons.

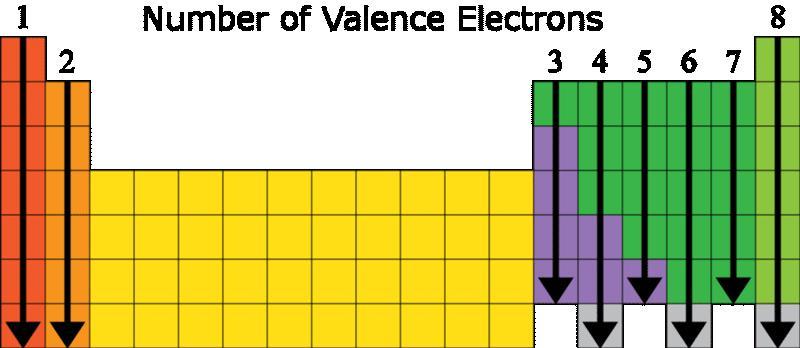
A single electron from the outermost shell of this element will move into the outermost shell of the atom with seven electrons.Nothing will happen in these state.
What is an electronic configuration ?Electron configurations define the arrangement of electrons around an atom's nucleus. Lithium, for instance, contains two electrons in the 1s subshell and one electron in the 2s subshell, according to its electron configuration, 1s22s1.
We may determine that Neon is the element with that electron configuration by looking at both of the tables below (Ne). It has all 8 electrons in its valence shell since it is on the extreme right side of the periodic table.
Noble gases are the elements on the right side of the periodic table that contain the most electrons and are thus non-reactive.
Thus, Electronic configuration an element is 1s22s22p6, it is stable.
To learn more about an electronic configuration, follow the link;
https://brainly.com/question/29757010
#SPJ5
help help help help help

Answers
Answer:
It's C.
Explanation:
According to the site, "http://needtoknow.nas.edu/energy/energy-sources/renewable-sources/wind/" ... "Wind energy is an indirect form of solar energy created by a combination of factors, including the uneven heating of Earth’s atmosphere by solar radiation, variations in topography, and the rotation of Earth. People have been putting wind energy to use throughout history to propel sail boats, mill flour from grain, and pump water."
4. Explain why diisopropylamine is a stronger base than aniline nh2 diisopropylamine aniline.
Answers
Answer: Cyclohexylamine is a stronger base than aniline because in aniline electron pair is involved in conjugation, which makes the electron pair unavailable, where as in cyclohexyl amine, the −NH
2
group is out side the ring and there is no resonance in the ring also, thereby the lone pair present on the nitrogen is freely available, resulting in a stronger base.
Explanation: I dont have one...
Which measure of a gas does the expression
nRT/p
represent? (1 point)
O molar mass
O volume
O number of moles
O gas constant
Answers
Answer:
A. volume
Explanation:
Generally the equation for the ideal gas is mathematically given as
PV=nRT
Where
P=pressure
V=volume
R=gas constant
n=Number of Moles
T=Temperature
Therefore
V=nRT/P
Option A
For more information on this visit
brainly.com/question/17756498
Sex cells are produced by the process of mitosis.
True
False
Answers
Answer:
True is the Answer.
Explanation:
True.
What are TWO control variables?
Case study: Tom was leaving work one day, when it was raining, and realized that the shoes he was wearing did not do a good job protecting him from getting his feet wet. On the next rainy day, he asked two friends to wear the same grey socks as him and a pair of their best water proof shoes, which were from different brands. Then they all went outside for about 30 minutes. When they came back inside, they took off the shoes and estimated what percentage of their socks got wet. They made a graph showing their results and found that the Columbia sneakers were the most effective at keeping water out because those socks got the least wet.
select only two
- type of shoe
- grey socks
- percentage of sock that got wet
- 30 min outside
- rainy day that shoes were tested on
- Columbia sneaker
Answers
Answer1234
Explanation:
Diamond is an allotrope of :
a. carbon b. sillica
c. phosphorus c. Nitrogen
Answers
Answer:
carbon
Explanation:
because it is an allotrope of carbon
Answer:
Carbon
Explanation:
its literaly carbon
How much work is done when 7 N of force is used to push a stalled motorbike 5 m?
Answers
Hey there!
========================================| Welcome to Physics |
Topic for Discussion - Work
========================================What is "work" when it comes to Physics?"
In physics, work is the energy transferred to or from an object via the application of force along a displacement. In short, it is the force applied to an object over distance.
The formula for work is \(W=Fs\).
\(W=work\)\(F=force\)\(s=displacement/distance\)Given the scenario above, there is "a force of 7 N on a stalled motorbike for a distance of 5 meters (m)". Now, we have to "determine the amount of work" that is being done during this
Knowing our formula for work, simply apply the given data in the scenario using the formula to determine the work being done.
\(W=Fs\)
\(F=7\)
\(s=5\)
\(W=7*5\)
\(W=35\)
Hence, the work being done in the given scenario would be 35.
__________________________________________________
Hope this helps! If you mark Brainliest, TYSM! :D
2. How many moles of hydrogen are produced from the reaction of 3.86 moles of zinc with an excess of
hydrochloric acid?
Answers
Answer:
as per given balanced equation one mole Zn produces 1mole Hydrogen
when reacted with excess acid.
So 3 moles Zn will give 3moles Hydrogen
Explanation:
According to the balanced chemical equation, one mole of Zn produces one mole of hydrogen . Therefore, 3.86 moles of Zn metal will produce 3.86 moles of hydrogen gas.
What is mole ratio ?Mole ratio refers to the ratio of the number of moles of one substance in a chemical reaction to the number of moles of another substance in the same reaction.
It is derived from the balanced chemical equation for the reaction, which indicates the relative amounts of each substance involved in the reaction.
Mole ratio is important in stoichiometry, which is the study of the quantitative relationships between reactants and products in a chemical reaction.
The given chemical reaction is written as follows:
Zn + 2 HCl ⇒ ZnCl₂ + H₂
According to the balanced chemical equation, one mole of Zn produces one mole of hydrogen . Therefore, 3.86 moles of Zn metal will produce 3.86 moles of hydrogen gas.
Find more on mole ratio :
https://brainly.com/question/15288923
#SPJ2
what happens to the humidity if the temperature drops and the amount of moisture in the air stays the same?
A. The humidity is not affected by temperature
B. The humidity increases
C. The humidity decreases
D. The humidity stays the same
Answers
Answer:
C The humidity decreases
Explanation:
So, if the temperature drops so the humidity decreases so the moisture dops from what it was before.
Determine
the stoichiometry of the chemical equation written
below:(3pt)
1C3H8 + 5O2 → 3CO2 + 4H2O
Answers
Stoichiometrically, the mole ratio of C3H8 to O2 to CO2 to H2O is 1:5:3:4.
According to the equation, the mole ratio of C3H8 to O2 is 1:5. This means that 1 mole of C3H8 will require 5 moles of O2 for complete reaction.
Also, 1 mole of C3H8 when completely burned in 5 moles O2, will produce 3 moles of CO2 and 4 moles of H2O.
More on stoichiometry can be found here: https://brainly.com/question/9743981?referrer=searchResults
Which of the following statements are true of the maintenance of blood pressure (BP)?
Answers
C. If blood pressure decreases, filtration in the kidneys decreases.
D. If more blood returns to the heart, the ventricles contract more forcefully to pump it out, and this raises blood pressure.
What is the maintenance of blood pressure?The maintenance of blood pressure is defined as the high use of sodium in your diet and the low use of potassium in your diet can cause hypertension. It is also important to use those foods which are free from fats. It is also important that we should use fruits, vegetables, and grains in excessive amounts for the maintenance of blood pressure and for a healthy diet.
High blood pressure 190/110 mm Hg can cause the main vital organs of the body like the brain, heart, and kidney too. There are three factors that contribute to blood pressure which are resistance, blood viscosity, and blood vessel diameter.
So we can conclude that blood pressure is the pressure of circulating blood against the walls of the blood vessels.
Learn more about Blood Pressure here: https://brainly.com/question/25149738
#SPJ1
Which of the following statements are true of the maintenance
of blood pressure (BP)? (Read carefully and select all of the correct statements.)
A. Norepinephrine stimulates vasoconstriction in skin, viscera, and skeletal muscles, all of which lower BP.
B. Aldosterone stimulates the reabsorption of Na+ ions by the kidneys, which lowers BP.
C. If BP decreases, filtration in the kidneys decreases.
D. If more blood returns to the heart, the ventricles contract more forcefully to pump it out, and this raises BP.
E. If BP decreases, the kidneys secrete the enzyme renin, which stimulates the secretion of epinephrine to prevent a further decrease.
F. The hormone ANP increases the excretion of K+ ions by the kidneys, which helps conserve water and raise BP.
G. The hormone ADH helps the kidneys conserve water and helps prevent a decrease in BP.
H. The elasticity of the large arteries helps decrease diastolic BP.
Hi can someone help me out with this question!

Answers
Answer:
Physical
Explanation:
You just melted the solid with heat, it has no new properties.
Which of the following is the best explanation for the fact that crushing increases the rate at which a solid dissolves in a liquid?
Select one:
a. The temperature of the solution is increased when the solid is crushed.
b. The surface area of the solute is increased when it is crushed.
c. Crushing increases the amount of solute present.
d. Crushing makes stirring the solution easier.
Answers
the density of a 19.0% by mass ethylene glycol (c2h6o2) solution in water is 1.02 g/ml .
Answers
Answer:
The density of the 19.0% by mass ethylene glycol solution in water is approximately 1.02 g/ml.
Explanation:
To solve this problem, we need to determine the density of the ethylene glycol solution, which is given as 19.0% by mass. Let's calculate the density of the solution.
Let's assume we have 100 grams of the ethylene glycol solution.
The mass of ethylene glycol in the solution is 19.0 grams (19.0% of 100 grams).
The mass of water in the solution is 81.0 grams (100 grams - 19.0 grams).
Now, we need to determine the volume of the solution. We can use the density of the solution, which is given as 1.02 g/ml.
Let V represent the volume of the solution in milliliters.Using the density formula:
Density = Mass / Volume, we can rearrange it to solve for Volume: Volume = Mass / Density.
For the ethylene glycol solution,
Volume = (19.0 grams + 81.0 grams) / 1.02 g/ml
= 100 grams / 1.02 g/ml
≈ 98.04 ml.
Therefore, the volume of the ethylene glycol solution is approximately 98.04 ml.
To obtain the density of the solution, we divide the total mass (100 grams) by the total volume (98.04 ml):
Density = 100 grams / 98.04 ml
≈ 1.02 g/ml.
Therefore, the density of the 19.0% by mass ethylene glycol solution in water is approximately 1.02 g/ml.
Learn more about density here, https://brainly.com/question/17780219
#SPJ11
In hospitals, staff members wear shoes with special soles to prevent build up of static charge as they walk. Why?Single line text.
Answers
Answer:
The correct answer is - sudden static charge could damage sensitive medical equipment.
Explanation:
Staff members of a hospital or medical institution wear special shoes that are able to prevent static charge as they walk. These shoes help to prevent static charge as we know that the sudden transfer of a static charge to medical equipment may alter the reports or damage sensitive equipment. This type of static charge is negative or positive due to electronic devices touches the device leads to damage of electric devices.
Thus, the correct answer is - sudden static charge could damage sensitive medical equipment.
give two example for desert plants and animals?
please mark as brainliest
Answers
Answer:
Plants : Cactus, tumbleweed
Animals : camel, fennec fox
Answer:
Desert plant - Cactus & Tumbleweed
Desert animal - Camel & Scorpion
We wish to determine how many grams
of solid silver chromate will precipitate
when 150. mL of 0.500 M silver nitrate
solution is added to excess potassium
chromate.
2AgNO3(aq)
How many moles of AgNO3 are present
in 150. mL of 0.500 M AgNO3?
+ K₂ CrO4 (aq) → Ag₂ CrO4(s) + 2KNO3(aq)
Answers
Approximately 12.45 grams of solid silver chromate will precipitate when 150 mL of 0.500 M silver nitrate solution is added to excess potassium chromate.
To determine the number of moles of AgNO3 present in 150 mL of a 0.500 M AgNO3 solution, we can use the formula:
moles = concentration × volume
Given:
Concentration of AgNO3 solution = 0.500 M
Volume of AgNO3 solution = 150 mL
First, we need to convert the volume from milliliters (mL) to liters (L) since the concentration is given in moles per liter (M).
1 L = 1000 mL
Therefore, the volume of the AgNO3 solution in liters is:
150 mL × (1 L / 1000 mL) = 0.150 L
Now we can calculate the moles of AgNO3 using the formula:
moles = concentration × volume
moles = 0.500 M × 0.150 L
moles = 0.075 mol
So, there are 0.075 moles of AgNO3 present in 150 mL of the 0.500 M AgNO3 solution.
Now, let's proceed to determine how many grams of solid silver chromate (Ag2CrO4) will precipitate when the AgNO3 solution reacts with excess potassium chromate (K2CrO4).
From the balanced chemical equation:
2AgNO3(aq) + K2CrO4(aq) → Ag2CrO4(s) + 2KNO3(aq)
We can see that the molar ratio between AgNO3 and Ag2CrO4 is 2:1. Therefore, for every 2 moles of AgNO3, we will form 1 mole of Ag2CrO4.
Since we have 0.075 moles of AgNO3, we can calculate the moles of Ag2CrO4 formed:
moles of Ag2CrO4 = 0.075 mol / 2 = 0.0375 mol
To determine the mass of Ag2CrO4, we need to multiply the moles by its molar mass. The molar mass of Ag2CrO4 is calculated by summing the atomic masses of each element in the compound:
Ag2CrO4 = 2(Ag) + 1(Cr) + 4(O) = 2(107.87 g/mol) + 1(52.00 g/mol) + 4(16.00 g/mol) = 331.87 g/mol
mass of Ag2CrO4 = moles of Ag2CrO4 × molar mass of Ag2CrO4
mass of Ag2CrO4 = 0.0375 mol × 331.87 g/mol = 12.45 g
For more such questions on solid silver chromate visit:
https://brainly.com/question/32055228
#SPJ8
why are mass and volume not properties of substances
Answers
Answer:
Extensive properties, such as mass and volume, depend on the amount of matter being measured. Intensive properties, such as density and color, do not depend on the amount of the substance present. Physical properties can be measured without changing a substance's chemical identity
Explanation:
Answer:
yes this above answer is right
Which object is at the center of the solar system in the heliocentric model?
A. The asteroid belt
B. The Sun
C. Earth
D. The Moon
Answers
In the heliocentric model, the object at the center of the solar system is the Sun. Therefore, the answer is B.
What is the heliocentric model?
The heliocentric model is a theory that places the Sun at the center of the solar system, with all the planets orbiting around it. This model was first proposed by the ancient Greek astronomer Aristarchus of Samos in the 3rd century BCE, but it was not widely accepted until the 16th century when the Polish astronomer Nicolaus Copernicus presented a detailed mathematical description of the heliocentric model.
The heliocentric model provided a more accurate description of the solar system, and it was later confirmed by the observations and calculations of other astronomers such as Johannes Kepler and Galileo Galilei. The heliocentric model is now widely accepted, and it forms the basis of modern astronomy.
To know more about the solar system, visit:
https://brainly.com/question/29608606
#SPJ1
The complete question is: The Sun is at the center of the solar system in the heliocentric model.