Which balanced chemical equation is a single-replacement reaction? (2 Points)
A. 2 K(s) + Br₂(/) → 2 KBr(s)
B. 2 HgO(s) → 2 Hg(/) + O₂(g)
C. Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
D. NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
Answers
Option D, NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s), is the balanced chemical equation that represents a single-replacement reaction.
What is Replacement Reaction?
In chemistry, a replacement reaction is a type of chemical reaction where one element replaces another in a compound. The general formula for a replacement reaction is:
A + BX → AX + B
where A and B are elements, and X is an anion that can combine with A and B. In this reaction, A replaces B in the compound BX, forming a new compound AX and releasing the element B as a free element. Replacement reactions can be of two types: single replacement reactions and double replacement reactions, depending on whether one or two elements are replaced in the reaction.
In this reaction, sodium (Na) replaces silver (Ag) in the compound AgNO3 to form NaNO3, and silver is released as a free element in the form of AgCl.
Learn more about Replacement Reaction from the given link
https://brainly.com/question/23918356
#SPJ9
Related Questions
compared to the polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water, the hydrogen bonds that hold multiple water molecules together are much stronger.
Answers
The polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water, the hydrogen bonds that hold multiple water molecules together are much stronger.
The above statement is True.
Compared to the polar covalent bonds that hold oxygen and hydrogen atoms together within a water molecule, the hydrogen bonds that hold a together are much stronger. A hydrogen bond is a type of intermolecular force that occurs between a positively charged hydrogen atom of one molecule add a negatively charged oxygen or nitrogen atom of another molecule. These bonds are much stronger than the typical dipole-dipole forces that occur between polar molecules and are a major contributor to water's unique physical and biological properties.
Multiple water molecules are held together by strong, which are much more stronger than the polar covalent relationships that keep the hydrogen and oxygen atoms elements together within a single water molecule. As weak attractive forces, hydrogen bonds are much weaker than covalent polar bonds.
As a result, the proton end of the molecule is partly positively charged, whereas the oxygenation end is partially negatively charged. By virtue of its covalently bonded bonding and curving shape, water is characterized as a polar covalent. Molecular water hydrogen bonds Water molecules are attracted to each other happily because the their polarity.
A hydrogen atom that is slightly will bind to a hydrogen atom that is slightly in a hydrogen bond. They could be detected between water molecules.
Complete Question:
Compared to the polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water, the hydrogen bonds that hold multiple water molecules together are much stronger. True/False ?
To know more about Molecules click here
brainly.com/question/19922822
#SPJ4
a school bus drives 35 mph down the street and slows as it approaches the stop sign

Answers
A student makes a cross-section clay model to represent the internal structure of a volcano that leads to the formation of two rocks (X and Y). The student records the processes that
affect the type of rock that is formed in a table,
volcano
aboveground
underground
K
Process
1
2
3
Details
rate of cooling of the rock
composition of the rock
pressure exerted on the rock
Which process(es) cause(s) the rock formed at location X to be different from the rock formed at location Y?
1 only
2 only
• 1 and 3
Activate Windows
Go to Settings to activate Windows.
2 and 3
Tas PM

Answers
CAnswer:
Explanation:
4.Identify each substance in the following word equation as a reactant or a product
limestone heat lime + carbon dioxide
5.What is the difference between an exothermic and endothermic reaction?
Answers
Explanation:
4. limestone heat lime + carbon dioxide
The reactants in this expression above is limestone
The products of the reaction is carbon dioxide and lime
Reactant is the species that gives the product and it is usually found on the left hand side of the expression.
The product is the substance on the right hand side of the expression that forms through the experiment.
Heat is used to facilitate the reaction.
5. An exothermic reaction is a reaction in which heat is given off.
An endothermic reaction is a reaction in which heat is absorbed in the process.
An exothermic reaction is always warmer after the reaction whereas an endothermic reaction is colder at the end of the reaction.
WHat are universal indicators and what are their uses
Answers
gentian violet is a dye using in dna gel electrophoresis it is yellow in strongly acidic solutions and purple in solutions ______
Answers
Gentian violet, a dye used in DNA gel electrophoresis, exhibits a yellow color in strongly acidic solutions and turns purple in solutions with higher pH levels, such as neutral or basic solutions. This color change aids in the visualization of DNA fragments during the gel electrophoresis process.
Gentian violet is a common dye used in DNA gel electrophoresis to stain DNA bands. It is a cationic dye that binds to DNA molecules, making them visible under UV light. Gentian violet appears yellow in strongly acidic solutions and purple in solutions with a higher pH. During electrophoresis, the DNA is separated by size and charge, resulting in distinct bands on the gel. Gentian violet stains these bands, allowing scientists to visualize the DNA fragments. However, excessive use of gentian violet can damage DNA, so it is important to use it in moderation. In summary, gentian violet is a vital tool for DNA analysis, but its use must be carefully controlled to prevent any negative effects on the DNA samples.
To know more about gel electrophoresis visit:
https://brainly.com/question/30791630
#SPJ11
If Block A is located on the Moon and Block B is located on
Earth, which of the following is true about the two blocks?
a They have the same mass.
sty
b They have the same weight.
ed a Hint?
C They have the same volume.
d They have the same density.
Answers
Answer: they have the same mass
Explanation:weight is calculated
How many mL in 5.867 L?
Answers
Answer:
5867ml is the answer to 5.867L to ml
HELP ME PLS
What is the mass of a water molecule if the hydrogens each have one neutron and the oxygen has eight neutrons?
a. 10
b. 12
c. 16
d. 20
Answers
Answer:
H2O
=(1×2)+(8)
=20
Explanation:
We have 1 Hydrogen(H) and 8 Oxygen(O)neutrons . Following the molecule formula, H2O, which means we have 2 Hydrogen that needs to times with 1 neutron;and we have only 1 Oxygen which has 8 neutrons. So 2 (H) have to times 1 and 1(O) have to times 8.
From which natural resource did John D. Rockefeller make his fortune? (1 point)
iron
O coal
O oil
O steel
Answers
Answer:
How did John D Rockefeller make his fortune? Rockefeller founded the Standard Oil Company in 1870. He ran it until 1897, and remained its largest shareholder.
Answer:
oil
Explanation:
PLEASE HELP
Which shows an isomer of the molecule below?
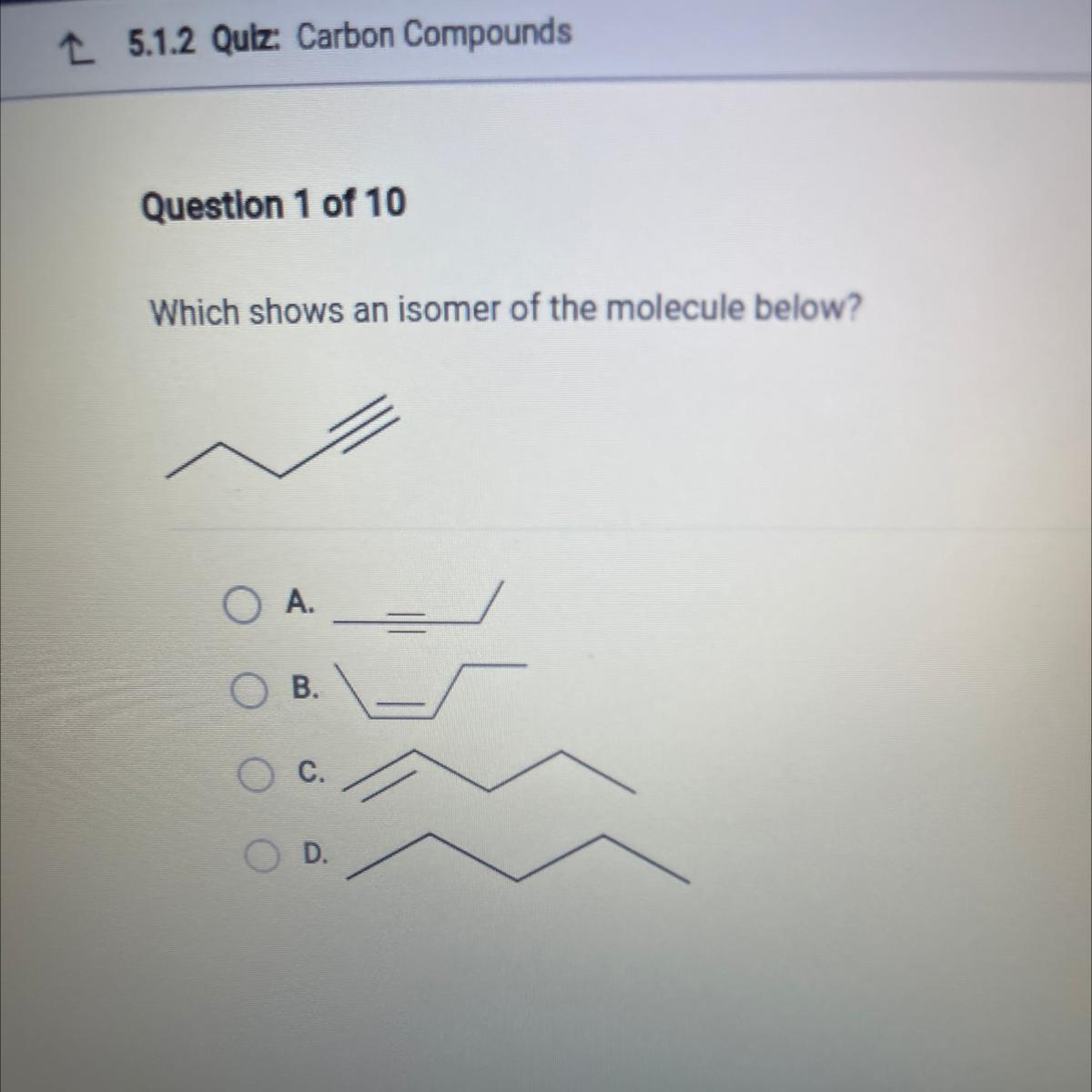
Answers
assuming a thermal to electric efficiency of 30% we want to run a 100 w light bulb for a year. (4 points) (a) using 235u, how much as would be consumed in that year (b) how much coal would be required given a thermal output of 25 gj/ton 1
Answers
a) 3.21 kg of uranium would be consumed in a year to run a 100 W light bulb for a year, assuming a thermal to electric efficiency of 30% using 235u.
b) 35 kg of coal would be required to run a 100 W light bulb for a year
We have;
Thermal to electric efficiency = 30%
Power of the light bulb = 100 W
Thermal output of coal = 25 GJ/tona)
Uranium that would be consumed in a year to run a 100 W light bulb for a year:
Energy consumed in a year by the light bulb = 100 W × 24 hours/day × 365 days/year
= 876,000 Wh
= 876 kWh
Electric energy produced from the thermal energy = (Thermal to electric efficiency / 100) × Energy consumed
electric energy produced from the thermal energy = (30 / 100) × 876 kWh
= 262.8 kWh
Amount of uranium consumed in a year = Electric energy produced from the thermal energy / Energy density of uranium
= 262.8 kWh / 81.8 GJ/t
= 0.00321 t
= 3.21 kg
Therefore 3.21 kg of uranium would be consumed in a year to run a 100 W light bulb for a year.
b) Coal that would be required to run a 100 W light bulb for a year given a thermal output of 25 GJ/ton:
Energy consumed in a year by the light bulb = 100 W × 24 hours/day × 365 days/year
= 876,000 Wh
= 876 kWh
Electric energy produced from the thermal energy = (Thermal to electric efficiency / 100) × Energy consumedElectric
energy produced from the thermal energy = (30 / 100) × 876 kWh = 262.8 kWh
Amount of coal required = Thermal energy required / Thermal output of coal
The thermal energy required = Electric energy produced from the thermal energy / (Thermal to electric efficiency / 100)
Thermal energy required = 262.8 kWh / (30 / 100) = 876 kWh
Amount of coal required = 876 kWh / 25 GJ/ton = 0.035 t = 35 kg
35 kg of coal would be required to run a 100 W light bulb for a year given a thermal output of 25 GJ/ton.
To know more about electric efficiency: brainly.com/question/29247736
#SPJ11
which of the following is false? group of answer choices applied pressure only refers to atmospheric pressure distillation separates compounds based on boiling point boiling point is the temperature at which the vapor pressure of the liquid equals the applied pressure increasing temperature increases vapor pressure
Answers
The false statement is applied pressure only refers to atmospheric pressure and the correct option is option 1.
It is the ratio of the force applied to the surface area over which the force is applied. Pressure can be defined as the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Atmospheric pressure is the pressure exerted by the atmosphere on the earth.
Thus applied pressure not only represents atmospheric pressure.
Learn more about Pressure, here:
https://brainly.com/question/29341536
#SPJ4
Some common substances and their chemical formulas are listed in the chart.
Which of these substances are elements?
Answers
Answer:
We heart you don't have all the information you just got some common substances and their chemical formulas are listed in the chart? That we don't have the chart to see which of the substances are elements
Explanation:
sorry about that if you can take a picture of it and repost it I am sure I could answer it for you
Please help, only a couple of days left!!!


Answers
Answer:
the heat from the universe during the big bang
a.k.a.
ITS A
Explanation:
the universe is still expanding, so what is gonna be used to create that new matter that's expanding the universe? energy and heat from the beginning of the universe! the universe started off as this point that was probably smaller than a quantum foam and infinite heat and started expanding from there
Get it right :D if this is wrong or not correct ill give u a bad review And no brainiest
Answers
Answer:
In short, because we are tearing up the oxygen factories to make way for carbon dioxide emitters. (Doesn't make a lot of sense, read the explanation)
Explanation:
So, 1000 years ago, we had a lot more trees, didn't have engines or cars or factories or anything, really that released carbon dioxide into the air and we had a lot more trees and since the invention of cars, engines, carbon dioxide-emitting tools and factories and all the other things that emit "Greenhouse gases" and in doing that, cleared more trees to make room for factories and roads and that has drastically changed the outlook of the carbon cycle.
Calculate the pH of 0.100 M NaCN. The K, for HCN is 9.12 x 10- Hint: You need to solve the problem using the quadra formula without approximation A. 2.98 B. 11.02 C. 4.52 D.9.48 E. 9.04
Answers
The given chemical equation is:H+ + CN- ⇋ HCN .The equilibrium constant is given as:Kc = [HCN]/([H+] [CN-])We know that the concentration of NaCN is equal to the concentration of CN-, the [CN-] is 0.100 M.
The formula to calculate pH is:pH = -log[H+]Initially, we need to calculate the concentration of H+ ions.Using the chemical equation, we can write:
[HCN] = [H+] [CN-]/KcLet [H+] be x, we have
[HCN] = x (0.100)/Kc[HCN]
= (x * 0.100)/9.12 x 10^-10
= (0.100x)/9.12 x 10^-10(x * 0.100)/(0.100x)
= 9.12 x 10^-10/0.100
= 9.12 x 10^-9
Now we can apply the quadratic formula to solve for x.
x2 + 9.12 x 10^-9 x - 9.12 x 10^-11 = 0x
= (-9.12 x 10^-9 ± √(9.12 x 10^-9)^2 - 4(1)(-9.12 x 10^-11))/(2(1))x
= (-9.12 x 10^-9 ± √8.34144 x 10^-17)/2
Now we need to select the correct value for x.pH = -log[H+]When we solve for x, we get two values:
x = 1.20 x 10^-4, -7.56 x 10^-10
Since the value of x cannot be negative, we select the positive value of x.
x = 1.20 x 10^-4pH
= -log[H+]pH
= -log[1.20 x 10^-4]pH
= 3.9
Therefore, the correct answer is option C. 4.52.
To know more about equilibrium , visit;
https://brainly.com/question/517289
#SPJ11
Since pH + pOH = 14, we can calculate the pH:
pH = 14 - pOH = 14 - 4.52 = 9.48
Therefore, the pH of the 0.100 M NaCN solution is approximately 9.48.
To calculate the pH of a solution of NaCN, we need to consider the dissociation of NaCN into Na+ and CN- ions. CN- can react with water to form HCN and OH- ions. The equilibrium expression for this reaction is:
CN- + H2O ⇌ HCN + OH-
Given that the K value for this reaction is 9.12 x 10^(-10), we can set up an equilibrium expression:
K = [HCN] * [OH-] / [CN-]
Since NaCN is a strong electrolyte, we can assume that the concentration of CN- after dissociation is equal to the initial concentration of NaCN, which is 0.100 M.
Let's assume that the concentration of OH- at equilibrium is x M. The concentration of HCN would also be x M, and the concentration of CN- would be 0.100 M - x M.
Plugging these values into the equilibrium expression, we have:
9.12 x 10^(-10) = (x * x) / (0.100 - x)
Simplifying the equation, we get:
9.12 x 10^(-10) = x^2 / (0.100 - x)
Multiplying both sides by (0.100 - x), we have:
9.12 x 10^(-10) * (0.100 - x) = x^2
Expanding and rearranging the equation, we obtain a quadratic equation:
9.12 x 10^(-11) - 9.12 x 10^(-10) * x + x^2 = 0
Now, we can solve this equation using the quadratic formula:
x = (-b ± √(b^2 - 4ac)) / (2a)
For this equation, a = 1, b = -9.12 x 10^(-10), and c = 9.12 x 10^(-11).
Solving the quadratic equation using the quadratic formula, we find two solutions for x:
x = 2.98 x 10^(-5) M (approximately) or x = 9.12 x 10^(-6) M (approximately)
The concentration of OH- at equilibrium is x, so the concentration of OH- is approximately 2.98 x 10^(-5) M.
Since pH is defined as the negative logarithm of the hydrogen ion concentration, we can calculate the pOH as:
pOH = -log10([OH-]) = -log10(2.98 x 10^(-5)) = 4.52
Finally, since pH + pOH = 14, we can calculate the pH:
pH = 14 - pOH = 14 - 4.52 = 9.48
Therefore, the pH of the 0.100 M NaCN solution is approximately 9.48.
To know more about pH, visit:
https://brainly.com/question/2288405
#SPJ11
How well does the number of beers a student drinks predict his or her blood alcohol content? Sixteen student volunteers at Ohio State University drank a randomly assigned number of cans of beer. Thirty minutes later, a police officer measured their blood alcohol content (BAC). The data are in the file p:\data\math\hartlaub\Elements of Statistics\bac.csv. The students were equally divided between men and women and differed in weight and usual drinking habits. Because of this variation, many students don't believe that the number of drinks predicts blood alcohol well.Make a scatterplot of the data. Find the equation of the least-squares regression line for predicting blood alcohol from number of beers and add this line to your plot. What is r-squared for these data? Briefly summarize what your data analysis shows.
Answers
Scatterplot: To visualize the relationship between these two variables, create a scatterplot of the number of beers versus blood alcohol content (BAC).
The next step would be to find the equation of the least-squares regression line, which would give the best prediction of BAC based on the number of beers. A regression analysis software or a statistical formula can be used to calculate the equation.
R-squared is the coefficient of determination, and it measures the strength of the linear relationship between two variables. A strong linear relationship is indicated by a high R-squared value, whereas a weak relationship is indicated by a low R-squared value.
The data analysis demonstrates the relationship between the number of beers and the BAC, as well as the accuracy of the prediction made using the least-squares regression line. The R-squared value provides information on the strength of this relationship, which helps determine the usefulness of using the number of beers to predict BAC.
It's important to note that this analysis should be interpreted with caution, as factors such as body weight, gender, and other individual characteristics can also affect BAC levels.
To Learn More About data analysis click
https://brainly.com/question/13103333
#SPJ4
Which device or substance is a type of nuclear reactor?.
Answers
A nuclear fission reactor is a device or substance that utilizes nuclear fission,
The process of splitting atomic nuclei, to generate heat and electrical power.
The reactor consists of a nuclear reactor core where the fission reactions occur.
Within the core, fuel rods containing isotopes such as uranium-235 (\(^{235}_{92}\tex{U}\)) or plutonium-239 (\(^{239}_{94}\tex{Pu}\)) serve as the nuclear fuel.
These isotopes undergo controlled fission reactions, releasing a tremendous amount of energy in the form of heat.
To prevent an uncontrolled chain reaction, control rods made of materials like boron or cadmium are inserted into the reactor core.
These rods absorb excess neutrons and help regulate the fission process.
The heat generated by the fission reactions is removed using a cooling system, typically involving the circulation of water or liquid sodium.
This heat is then transferred to a secondary system, where it is used to produce steam. The steam drives a turbine, which converts the thermal energy into mechanical energy.
A generator converts this mechanical energy into electrical energy.
Nuclear fission reactors play a significant role in generating electricity in nuclear power plants.
They provide a reliable and efficient source of power while minimizing greenhouse gas emissions.
The use of nuclear fuel, such as uranium-235 and plutonium-239,
Ensures a sustained supply of energy, making nuclear fission reactors an essential component of the global energy infrastructure.
To know more about Nuclear reactor here: https://brainly.com/question/32814463
#SPJ11
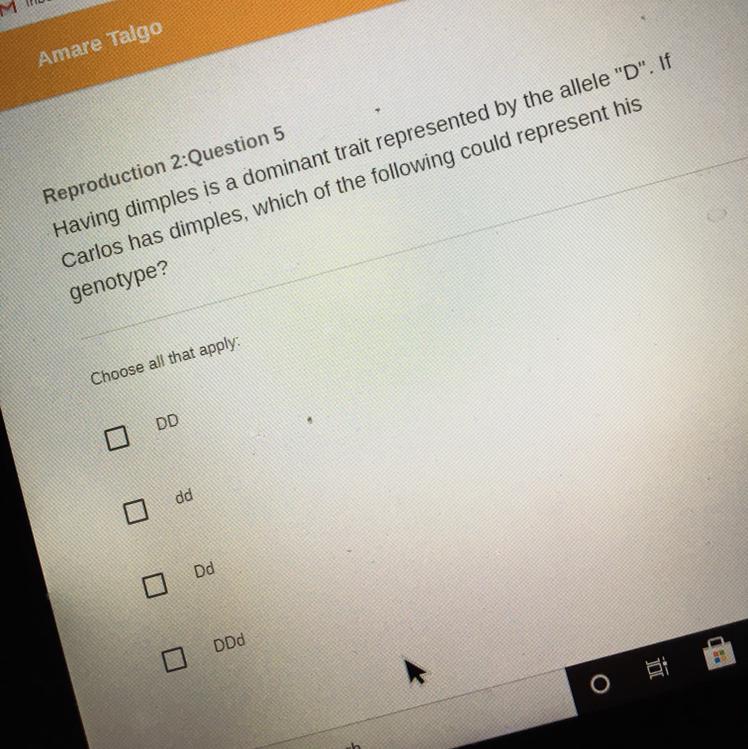
science , thank you for helping !!
Answers
Answer:
sksksk
Explanation:
anna oop
Answer:
Try putting Dd okkkkkkkk
A piston at 27.0 °C, 8.5 L, and 14.6 psi pressure, has its pressure change to 103.8 kPa. What change must have occurred to the volume to cause this type of pressure change?
Answers
The volume decreased by 1.3 L (from 8.5 L to 7.2 L) to cause the pressure change from 14.6 psi to 103.8 kPa at constant temperature. To determine the change in volume that caused the pressure change, we can use the ideal gas law:
PV = nRT
where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature.
First, we need to convert the initial pressure and volume to SI units:
P1 = 14.6 psi = 100.68 kPa
V1 = 8.5 L
Next, we can use the ideal gas law to calculate the number of moles of gas in the initial state:
n1 = (P1 V1) / (RT1)
where R is the ideal gas constant (0.0821 L·atm/K·mol) and T1 is the temperature in Kelvin (27.0 + 273 = 300 K).
Similarly, we can use the ideal gas law to calculate the number of moles of gas in the final state:
n2 = (P2 V2) / (RT2)
where P2 is the final pressure (103.8 kPa), T2 is the temperature (also 27.0 °C + 273 = 300 K), and we want to solve for V2, the final volume.
Equating the number of moles of gas in the two states (since the amount of gas remains constant):
n1 = n2
(P1 V1) / (RT1) = (P2 V2) / (RT2)
Solving for V2, we get:
V2 = (P1 V1 RT2) / (P2 RT1)
Substituting the given values, we get:
V2 = (100.68 kPa x 8.5 L x 300 K) / (103.8 kPa x 0.0821 L·atm/K·mol x 300 K)
V2 = 7.2 L
Therefore, the volume decreased by 1.3 L (from 8.5 L to 7.2 L) to cause the pressure change from 14.6 psi to 103.8 kPa at constant temperature.
To know more about ideal gas law, visit:
https://brainly.com/question/28257995
#SPJ1
PLEASE HELP WITH #1 AND #2 ASAP!! PLEASE

Answers
The rate constant for this first‑order reaction is 0.0830 s−1
at 400 ∘C.
A⟶products
After how many seconds will 16.8%
of the reactant remain?
Answers
The reaction has not yet started, and the time required for 16.8% of the reactant to remain cannot be calculated using the given rate constant.
Given,The rate constant for this first-order reaction is 0.0830 s−1 at 400 ∘C.A⟶products After how many seconds will 16.8% of the reactant remain-The time taken for a first-order reaction to reach a particular percentage of completion can be calculated using the following formula:t = (ln(A/A₀))/kwhere A₀ is the initial concentration of the reactant, A is the concentration of the reactant at a given time, k is the rate constant, and t is the time elapsed since the reaction began.In this question, we are given the rate constant, k = 0.0830 s−1 at 400 ∘C, and we want to find out the time required for 16.8% of the reactant to remain.Let's assume that the initial concentration of the reactant is 100 units (we can assume any value as it does not affect the percentage of completion).Therefore, the concentration of the reactant remaining after 16.8% of completion would be: A = 16.8 units.Substituting these values in the above formula, we get:t = (ln(16.8/100))/0.0830t = (−1.7918)/0.0830t = −21.58 sThis time value is negative, which means that the reaction has not even started yet. Therefore, we need to check the given percentage of completion.
If it is less than 50%, we can assume that the reaction has not yet started. In this case, the percentage of completion is 16.8%, which is less than 50%.
for such more questions on reaction
https://brainly.com/question/24795637
#SPJ8
Convert 0.30 m to mm.
Answers
Answer:
0.3 Meters = 300 Millimeters
Explanation:
Multiply the length value by 1000
Hopefully, this helps! :D
why does the sky appear orang or red at sunset and sunrise?
Answers
Answer:
Because the sun is low on the horizon, sunlight passes through more air at sunset and sunrise than during the day, when the sun is higher in the sky. More atmosphere means more molecules to scatter the violet and blue light away from your eyes.
The noble gas thought to be significantly carcinogenic due to its radioactive decay and that of its decay products is.
Answers
The answer is radon. Radon is a colorless and odorless gas that is formed naturally from the decay of uranium and thorium in soil, rock, and water. Radon is considered significantly carcinogenic because it emits alpha particles, which can damage the DNA in our cells and lead to cancer.
When inhaled, radon and its decay products can cause lung cancer, especially in people who are exposed to high levels over a long period of time.
In terms of its radioactivity, radon has a half-life of 3.8 days, which means that half of a given amount of radon will decay in that time. However, its decay products, such as polonium-218 and lead-214, also emit alpha particles and have longer half-lives. These decay products can attach to dust and other airborne particles, which can be inhaled and increase the risk of lung cancer.
In summary, radon is the noble gas that is significantly carcinogenic due to its radioactive decay and that of its decay products. It is important to test for radon levels in homes and workplaces and to take steps to reduce exposure if levels are found to be high.
To know more about DNA visit:
brainly.com/question/264225
#SPJ11
What do you infere about using nonrenweable vs renewable resources and the effect this has on the cost of energy
Answers
Answer:
See explanation
Explanation:
Renewable energy resources refers to energy resources that can not be depleted such as wind, solar, biomass etc.
Non-renewable energy resources refers to those energy resources that can be depleted such as fossil fuels.
Globally, it has been established that energy from renewable sources costs less than energy from nonrenewable(especially fossil fuel) sources.
According to Forbes, 2019, "The worldwide weighted average cost of electricity from solar power concentration fell by 26%, that of bioenergy reduced by 14%, solar photovoltaics, geothermal, onshore, as well as offshore wind, fell by 14%, and hydropower by 12%.".
This shows us that renewable energy remains the cheapest alternative in terms of energy cost.
What holds more salt warm or cold water?
Answers
I have a question, about trends in the periodic table. Mostly focusing on ionization energy. From going from right to left it increases as the atom's size decreases. Can someone explain why this happens? Like why does it increase as the size decreases Thank you.
Answers
It consists of two lobes that contain pollen sacs and pollen grains.
Answers
Answer:
Assuming you need the names :)
Explanation:
It's dithecous anther