Which atom or ion has the smallest atomic radius?
(a) Li
(b) Li+
(c) Mg
(d) Mg2+
(e) Al
(f) Al3+
Answers
The Al3+ ion has a smaller atomic radius compared to neutral aluminium (Al) or other ions listed.
What is atomic radius?An atomic radius is half the distance between adjacent atoms of the same element in a molecule.
Measuring the atomic radii of chemical elements is a complicated task as the size of an atom is of the order of 1.2 × 10 - 10 m. The electron cloud forming the shell of an atom does not have any fixed shape which makes it difficult to determine the atomic size of an atom. So we can say that practically we cannot determine the size of an individual atom.
The atomic radius generally decreases as you move across a period from left to right in the periodic table and increases as you move down a group.
Among the given options, the atom or ion with the smallest atomic radius would be (f) Al3+. When an atom loses electrons to become an ion with a positive charge, such as Al3+, it becomes smaller because there are fewer negatively charged electrons to balance the positive charge of the nucleus.
Therefore, the Al3+ ion has a smaller atomic radius compared to neutral aluminium (Al) or other ions listed.
Learn more about atomic radius
https://brainly.com/question/13963619
#SPJ4
Related Questions
which is the most accurate indicator of fluid volume status in the oliguric or anuric client?
Answers
Central venous pressure (CVP). CVP monitoring offers a more accurate and direct evaluation of their fluid volume status.
The most accurate indicator of fluid volume status in oliguric or anuric clients is daily weight measurement due to its ability to reflect fluid loss or gain.
Other indicators may provide important information but weight measurement is the main answer to the question.
CVP measures the pressure within the major veins returning blood to the heart, and it provides a reliable assessment of fluid volume status.
In oliguric or anuric clients, urine output is minimal or absent, making it difficult to determine their fluid balance using urine output alone.
CVP monitoring offers a more accurate and direct evaluation of their fluid volume status.
Summary: In clients with oliguria or anuria, the central venous pressure (CVP) is the most accurate indicator of their fluid volume status.
Learn more about veins click here:
https://brainly.com/question/4356549
#SPJ11
A twin-turbojet airplane is cruising with a speed of Mach 1.5 at an altitude where atmospheric pressure is 32989.5 Pa, temperature is 232.778 K. Each engine is consuming 200 kg of air per second. The engine exit flow has a pressure of 32,000 Pa with a velocity of 850 m/sec. The exit area of the engine nozzle is 1.4 m
2
. How much thrust both engines are generating?
Answers
A twin-turbojet airplane is cruising with a speed of Mach 1.5 at an altitude where atmospheric pressure is 32989.5 Pa, temperature is 232.778 K. Each engine is consuming 200 kg of air per second. The engine exit flow has a pressure of 32,000 Pa with a velocity of 850 m/sec. The exit area of the engine nozzle is 1.4 m². The thrust generating in both engines is 342,678.6 Newtons.
To calculate the thrust generated by both engines, we can use the momentum equation for a nozzle:
Thrust = mass flow rate * exit velocity + (exit pressure - ambient pressure) * exit area
Given:
Speed of the airplane (V) = Mach 1.5
Atmospheric pressure (\(P_a\)) = 32989.5 Pa
Ambient temperature (\(T_a\)) = 232.778 K
Mass flow rate of each engine (m) = 200 kg/s
Exit pressure of the engine (\(P_e\)) = 32000 Pa
Exit velocity of the engine (\(V_e\)) = 850 m/s
Exit area of the engine nozzle (\(A_e\)) = 1.4 m²
First, we need to calculate the ambient density using the ideal gas law:
PV = nRT
Since the speed of the airplane is given in terms of Mach number, we can calculate the speed of sound (a) using the following formula:
a = √(gamma * R * \(T_a\))
Where gamma is the specific heat ratio of air (approximately 1.4) and R is the specific gas constant for air (approximately 287 J/(kg K)).
Next, we can calculate the ambient density (ρ) using the equation:
ρ = \(P_a / (R * T_a)\)
Now, we can calculate the thrust generated by each engine using the momentum equation:
Thrust = m* \(V_e + (P_e - P_a) * A_e\)
Finally, we can calculate the total thrust generated by both engines by multiplying the thrust of a single engine by 2.
Calculate the speed of sound:
a = √(1.4 * 287 * 232.778)
a = 438.95 m/s
Calculate the ambient density:
ρ = 32989.5 / (287 * 232.778)
ρ = 1.383 kg/m³
Calculate the thrust of a single engine:
\(Thrust_s\) = 200 * 850 + (32000 - 32989.5) * 1.4
\(Thrust_s\) = 170000 + 1339.3
\(Thrust_s\) 171339.3 N
Calculate the total thrust of both engines:
\(Thrust_t\) = 2 * \(Thrust_s\)
\(Thrust_t\) = 2 * 171339.3
\(Thrust_t\) = 342678.6 N
Therefore, both engines are generating approximately 342,678.6 Newtons of thrust.
To know more about thrust here
https://brainly.com/question/26712174
#SPJ4
How many moles of hydrogen do you need to react with 0.85 moles of nitrogen?
Answers
Answer:
6 moles
Explanation:
You have a 1:3 ratio between nitrogen gas and hydrogen gas
you have 0.575 moles of each of the following elements: c, cl, ca, cr, and cd. which sample has the greatest mass?
Answers
The sample with the greatest mass is Cd, with a mass of 64.6 g. Molar mass is defined as the mass of one mole of a substance, usually expressed in grams per mole (g/mol).
We have 0.575 moles of each of the following elements: C, Cl, Ca, Cr, and Cd. The molar masses of these elements are:C: 12.01 g/molCl: 35.45 g/molCa: 40.08 g/molCr: 52.00 g/molCd: 112.41 g/mol
To find the mass of each sample, we can use the following formula:mass = moles x molar mass. We can calculate the mass of each sample as follows:C: 0.575 mol x 12.01 g/mol = 6.9 g, Cl: 0.575 mol x 35.45 g/mol = 20.3 g, Ca: 0.575 mol x 40.08 g/mol = 23.0 g , Cr: 0.575 mol x 52.00 g/mol = 29.9 g, Cd: 0.575 mol x 112.41 g/mol = 64.6 g. Therefore, the sample with the greatest mass is Cd, with a mass of 64.6 g.
For more such questions on Molar mass
https://brainly.com/question/837939
#SPJ8
PLEASE ASAP
HELP!!!!!!!!

Answers
Answer:
sec explanation: It is because it goes by order sec, min, day, month, and year etc
Answer:
sec
Explanation:
What are the half-reactions for a galvanic cell with Zn and Mg electrodes?
Answers
the half-reactions
cathode : Zn²⁺ (aq) + 2e⁻ ---> Zn (s)
anode : Mg (s) → Mg²⁺ (aq) + 2e−
a balanced cell reaction
Zn²⁺(aq) + Mg(s)→ Zn(s) + Mg²⁺ (aq)
Further explanationGiven
Zn and Mg electrodes
Required
The half-reactions for a galvanic cell
Solution
To determine the reaction of a voltaic cell, we must determine the metal that serves as the anode and the metal that serves as the cathode.
To determine this, we can either know from the standard potential value of the cell or use the voltaic series
1. voltaic series
Li-K-Ba-Ca-Na-Mg-Al-Mn- (H2O) -Zn-Cr-Fe-Cd-Co-Ni-Sn-Pb- (H) -Cu-Hg-Ag-Pt-Au
The more to the left, the metal is more reactive (easily release electrons) and the stronger reducing agent
So the metal on the left will easily undergo oxidation and function as anode
Since Mg is located to the left of Zn, then Mg functions as anode and Zn as a cathode
2. Standard potentials cell of Mg and Zn metals :
Mg2+ + 2e– → Mg E° = -2,35 V
Zn2+ + 2e– → Zn E° = -0,78 V
The anode has a smaller E°, then Mg is the anode and Zn is the cathode.
Answer:
Explanation:help
PLZ HELP WILL GIVE U BRANILEIEST
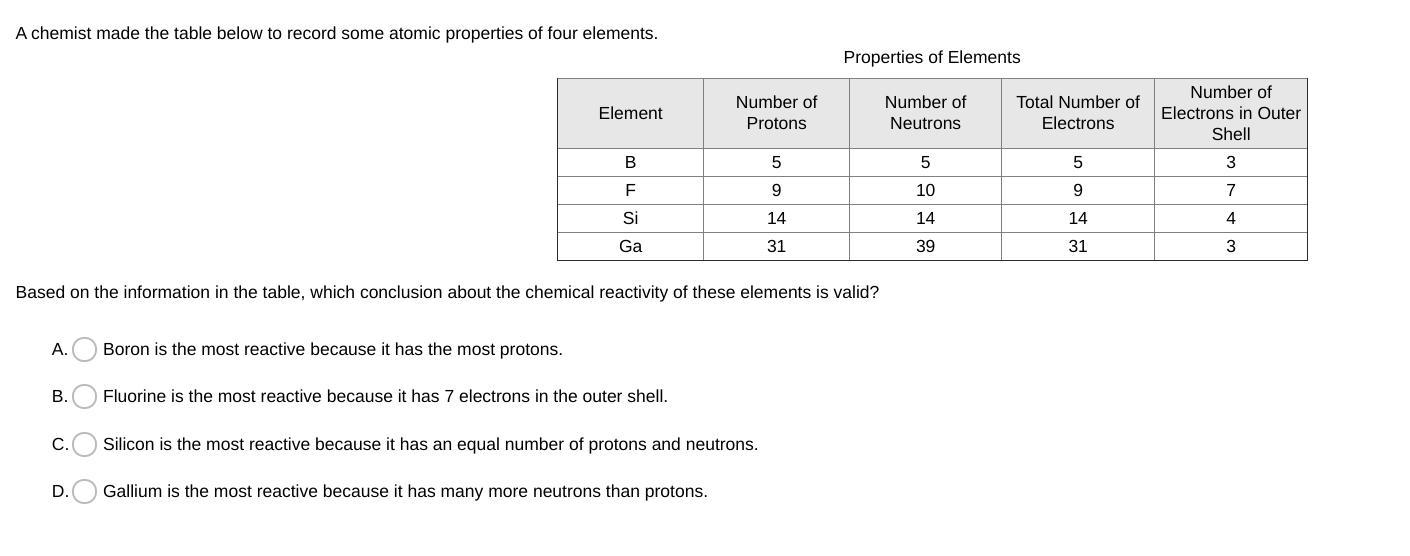
Answers
Answer:
I'm pretty sure it is C
Explanation:
because it has the same number of protons and neutrons
A Freon leak in the air-conditioning system of a large building releases 20kg of CHF2CL per month.
If the leak were allowed to continue, how many kilograms of Cl would be emitted into the atmosphere each year?
Answers
Approximately 8,199.30 grams or 8.1993 kg of Cl would be emitted into the atmosphere each year if the Freon leak were allowed to continue.
To determine the amount of Cl (chlorine) emitted into the atmosphere each year due to the Freon leak, we need to consider the molar mass and molecular structure of CHF2Cl (Freon-22).
The molar mass of CHF2Cl is approximately 86.47 g/mol. Given that 20 kg of CHF2Cl is released per month, we can convert it to grams: 20 kg * 1000 g/kg = 20,000 g.
Next, we calculate the number of moles of CHF2Cl: 20,000 g / 86.47 g/mol = 231.31 mol.
Each molecule of CHF2Cl contains one chlorine atom, so the number of moles of Cl is the same as the number of moles of CHF2Cl, which is 231.31 mol.
To convert moles to grams, we multiply by the molar mass of chlorine (35.45 g/mol): 231.31 mol * 35.45 g/mol = 8,199.30 g.
Therefore, approximately 8,199.30 grams or 8.1993 kg of Cl would be emitted into the atmosphere each year if the Freon leak were allowed to continue.
Know more about molar mass here
https://brainly.com/question/12127540#
#SPJ11
balance the following equation in acidic solution:mno4-(aq) as4o6(s) → mn2 (aq) aso43-(g)what is the coefficient of water?
Answers
The balanced chemical equation is given as
MnO₄⁻(aq) + As₄O₆(s) + 7H₂O(l) → Mn²⁺(aq) + AsO₄³⁻(g) + 14H⁺(aq) + 5e⁻
The coefficient of water (H₂O) is 7.
To balance the equation in acidic solution:
MnO₄⁻(aq) + As₄O₆(s) → Mn²⁺(aq) + AsO₄³⁻(g)
We can balance the atoms one by one:
Balance the atoms other than oxygen and hydrogen:
MnO₄⁻(aq) + As₄O₆(s) → Mn²⁺(aq) + AsO₄³⁻(g)
Balance oxygen atoms by adding H₂O:
MnO₄⁻(aq) + As₄O₆(s) + 7H₂O(l) → Mn²⁺(aq) + AsO₄³⁻(g)
Balance hydrogen atoms by adding H⁺ ions:
MnO₄⁻(aq) + As₄O₆(s) + 7H₂O(l) → Mn²⁺(aq) + AsO₄³⁻(g) + 14H⁺(aq)
Balance the charge by adding electrons (e⁻):
MnO₄⁻(aq) + As₄O₆(s) + 7H₂O(l) → Mn²⁺(aq) + AsO₄³⁻(g) + 14H⁺(aq) + 5e⁻
Now the equation is balanced. The coefficient of water (H₂O) is 7.
Learn more about balanced chemical equation from the link given below.
https://brainly.com/question/14072552
#SPJ4
Pretend your friend was absent from class today...
Write what you would say if you had to explain how and why elements to
your friend.
Answers
Answer:
how and why elements what?? why they exist?? im gonna assume that's what you meant, so elements exist because we're all on earth and without elements, we don't have resources to live. the earth is made of elements and if there were none, we wouldn't even have a planet
Explanation:
^
a phenol has an -oh group bonded to a(n) ________. disubstituted carbon singly substituted or unsubstituted carbon tetrasubstituted carbon carbon in a benzene ring trisubstituted carbon
Answers
A phenol has an -OH group bonded to a carbon in a benzene ring.
The structure of phenol consists of a benzene ring with a hydroxyl group (-OH) attached to one of the carbon atoms in the ring. This carbon atom is known as a carbon in a benzene ring, which is a sp2 hybridized carbon.
In the benzene ring, each carbon atom forms three sigma bonds with adjacent carbon atoms and one sigma bond with a hydrogen atom. The remaining electron in each carbon atom is part of the delocalized pi system, creating a planar aromatic structure.
The hydroxyl group (-OH) in phenol is directly bonded to one of the carbon atoms in the benzene ring, replacing one of the hydrogen atoms. This carbon is considered to be in a benzene ring because it is part of the aromatic system.
The presence of the -OH group attached to the benzene ring gives phenol its characteristic properties and reactivity, including its ability to undergo various chemical reactions such as hydrogen bonding, substitution reactions, and oxidation reactions.
Therefore, the answer is carbon in a benzene ring.
know more about Benzene Ring here:
https://brainly.com/question/31490176
#SPJ11
assign each species on the left to a category on the right. clear all naoh ch3cooh hi c5h11n strong acid weak acid strong base weak base
Answers
To assign each species to a category on the right, we need to understand the properties of each term.
- Strong acids are defined as substances that completely dissociate in water to form H+ ions. Examples of strong acids include HCl, HNO3, and H2SO4.
- Weak acids are defined as substances that only partially dissociate in water to form H+ ions. Examples of weak acids include CH3COOH and H2CO3.
- Strong bases are defined as substances that completely dissociate in water to form OH- ions. Examples of strong bases include NaOH and KOH.
- Weak bases are defined as substances that only partially dissociate in water to form OH- ions. Examples of weak bases include NH3 and C5H11N.
With these definitions in mind, we can now assign each species to a category on the right:
- NaOH: This is a strong base because it completely dissociates in water to form Na+ and OH- ions.
- CH3COOH: This is a weak acid because it only partially dissociates in water to form H+ and CH3COO- ions.
- HI: This is a strong acid because it completely dissociates in water to form H+ and I- ions.
- C5H11N: This is a weak base because it only partially dissociates in water to form OH- ions.
- Clear: This term is not a chemical species and cannot be assigned to any category.
Note: It's important to understand the definitions and properties of each term in order to correctly assign species to their respective categories.
Learn more about dissociate here:
brainly.com/question/30983331
#SPJ11
For any experiment involving solvent extraction, which of the following solvent physical properties must be included in your lab notebook pre-lab write-up? this physical property is essential for proper layer identification after phase separation.
Answers
Solvent extraction, or liquid-liquid extraction, is a commonly employed technique for separating specific compounds from a mixture.
When conducting experiments involving solvent extraction, it is crucial to include the density of the solvent as a physical property in the pre-lab write-up of your lab notebook.
Solvent extraction, or liquid-liquid extraction, is a commonly employed technique for separating specific compounds from a mixture.
This method involves shaking the mixture containing two immiscible liquids, allowing them to separate into distinct layers, and subsequently isolating the desired compound.
The density of the solvents plays a vital role in solvent extraction experiments. It determines the layer identification after phase separation.
By understanding the relative densities of the two liquids involved, we can predict which liquid will be the top layer and which will be the bottom layer.
The less dense liquid will float on top, while the more dense liquid will sink to the bottom.
This knowledge enables us to determine the layer that contains the target compound of interest.
Including the density of the solvents in the pre-lab write-up ensures accurate layer identification during the solvent extraction process.
It is an essential physical property that aids in the successful isolation of desired compounds.
To know more about Solvent extraction here: https://brainly.com/question/25418695
#SPJ11
Given the balanced equation:
2Al(s) + 6H+ (aq) -----> 2Al^3+ (aq) + 3H2(g)
What is the total number of moles of electrons gained by H+ (aq) when 2 moles of Al(s) are completely reacted?
1) 6
2) 2
3) 3
4) 12
Answers
The total number of moles of electrons transferred from Al to H⁺ is 6
The given reaction can be split up into two half-reactions as follows:
2 Al (s) → 2 Al³⁺ (aq) + 6 e⁻
6 H⁺ (aq) + 6 e⁻ → 3 H₂ (g)
From the first half-reaction, it is clear that 2 moles of Al give out 6 moles of electrons to become Al³⁺.
From the second half reaction, it is clear that these 6 moles of electrons are accepted by H⁺ to become H₂ gas.
What is a half-reaction?
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction
How does half-reaction work?
In general, the half-reactions are first balanced by atoms separately. Electrons are included in the half-reactions. These are then balanced so that the number of electrons lost is equal to the number of electrons gained. Finally, the two half-reactions are added back together.
To know more about half-reactions:
https://brainly.com/question/24082438
#SPJ1
Answer:
3
Explanation:
because i did this when i was a kid
n each reaction box, place the best reagent and conditions from the list.
You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop.
Answer Bank
NH3NaBH4, CH3CH2OH
PBr3, pyridine
NaNH2Br2, FeBr3CH3NH2H2SO4Mg, Et2OZn(Hg), HClCH3CNCH3MgBrNaCN
H2, Pt
In each reaction box, place the best reagent and conditions from the list.
You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop.
Answer Bank
CH3CNCH3NH2NaBH4, CH3CH2OH
PBr3, pyridine
CH3MgBrNaNH2Mg, Et2OH2SO4H2, PtNH3NaCNZn(Hg), HCl
Br2, FeBr3
Answers
Three stages are required to transform benzaldehyde into 2-phenyl acetonitrile.
A benzene ring with a formyl substituent makes up the chemical molecule known as benzoaldehyde. It is both the most readily used industrially and the most straightforward aromatic aldehyde. A benzene ring with a formyl substituent makes up the chemical molecule known as benzoaldehyde (C6H5CHO). It is both the most readily used industrially and the most straightforward aromatic aldehyde. It is a clear liquid with a distinct almond-like scent.
In addition to batteries, acetonitrile is used to create perfumes, rubber goods, insecticides, acrylic nail removers, and medications. Fatty acids are also extracted from both vegetable and animal oils using this method. Employees should receive safe handling and storage instructions prior to working with acetonitrile.
learn more about benzoaldehyde here
https://brainly.com/question/13263721
#SPJ4

Given the following laboratory observation, which of the following statements is NOT TRUE?Zn + CuBr2 à Cu + ZnBr2Cu + ZnBr2à No Reaction
Answers
The table below gives the numbers of protons, electrons, and neutrons in four atoms.
Atom
1
2
3
4
Number of protons
9
99
19
Based on the table, which atom has a charge of -1?
01
02
3
▸
Number of neutrons
8
19
19
10
Number of electrons
9
8
10
19
Answers
The numbers of protons, electrons, and neutrons in four atoms. Atom 1 has charge of -1.
Atom 1 2 3 4
Number of protons 9 9 9 19
Number of neutrons 8 19 19 10
Number of electrons 9 8 19 19
An atom is a particle of remember that uniquely defines a chemical element. An atom consists of a important nucleus this is surrounded by using one or extra negatively charged electrons. The nucleus is definitely charged and carries one or extra exceedingly heavy particles known as protons and neutrons.
An atom is any particle of count number at its most simple level which contains at the least one proton. here are a few examples of the atoms: hydrogen (H) and neon (Ne). However in terms of the phrase atom, we ought to go to historic Greece of four hundred B.C. And there has been a first rate truth seeker named Democritus, and he proposed the Greek word atomos, which means that uncuttable. And so as he defined, all count become ultimately reducible to discrete, small debris or atomos.
Learn more about atom here:-https://brainly.com/question/17545314
#SPJ1
Provide a set of step by step instructions to make 750 mL of 1.5 M calcium chloride solution.
Answers
Answer: PLease see answer in explanation column
Explanation:
FIRST STEP
We find the grams ( mass ) of solute required to prepare the solution by using the formula
grams ( mass ) of solute, g(CaCl2)= Molar mass x Molarity of the solution x Volume of the solution
Therefore Preparing 750 mL of 1.5 M calcium chloride solution, we have that Molar mass of Calcium chloride =110.98 g/mol
g(CaCl2) =110.98 g/mol X 1.5mol/L X 0.75L
g(CaCl2) =124.8525g CaCl2
SECOND STEP
Now Dissolve 124.8525g CaCl2 in about 350ml of distilled water then add more water until the final volume be comes 750ml
calculate the number of moles present in 9.50g of CO2
Answers
Answer: 44.0095.
Explanation:
How might the reversal of earths magnetic poles affect life on earth?
Answers
Answer:
If that happened it would weaken our protective magnetic field, which wouldn't be able to protect us from the harmful radiations sent through space. This could cause alot of issues like; damage cells, cause cancer, and fry electronic circuits and electrical grids.
Explanation:
Earths magnetic field is what protects us from harmful radiation!
Hope this helps! :)
This is what has happened when the magnetic poles flipped in the past. This could weaken Earth's protective magnetic field b up to 90% during a polar flip. Earth's magnetic field is what shields us from harmful space radiation which can damage calls, cause cancer, and fry electronic circuits and electrical grids.
HELP IS IT:
A.
Model 2 is an isotope; Model 3 is an ion
B.
Model 1 is an ion; Model 3 is an isotope
C.
Model 1 is an isotope; Model 2 is an ion
D.
Model 1 is an ion; Model 2 is an isotope

Answers
Answer:
i thjink its b
Explanation:
someone please help with number 14 letters a-d i don’t understand it!!

Answers
MgCO3⇒ MgO+CO2 is the balance equation reaction.
a. Li+O2⇒ LiO2
b. H2+Cl2⇒ H2Cl2
c.MgCO3⇒ MgO+CO2
d.2NaI+Cl2⇒ 2NaCl+2I
To balance chemical equations, stoichiometric coefficients for the reactants and products are required. This is important because a chemical equation must follow the rules of conservation of mass and constant proportions, which dictate that the same number of atoms of each element must be avail on two of the reactant and product sides of the equation.
Chemical equations use the relevant chemical formulae to symbolically express the reactants and products of a chemical process. To the left of the sign "," and to the right of the arrow symbol, respectively, is the part of the chemical equation that is on the reactant side.
To know more about balance equation visit : brainly.com/question/28949107
#SPJ1
Enter a complete ionic equation to show the reaction of aqueous Hg2(NO3)2 with aqueous sodium chloride to form solid Hg2Cl2 and aqueous sodium nitrate. Express your answer as a complete ionic equation. Identify all of the phases in your answer.
Answers
Answer: \(Hg_2^{2+}(aq)+2NO_3^-(aq)+2Na^+(aq)+2Cl^-(aq)\rightarrow Hg_2Cl_2(s)+2Na^+(aq)+2NO_3^-(aq)\)
Explanation:
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
A double displacement reaction in which one of the product is formed as a solid is called as precipitation reaction.
The balanced chemical equation is:
\(Hg_2(NO_3)_2(aq)+2NaCl(aq)\rightarrow Hg_2Cl_2(s)+2NaNO_3(aq)\)
The complete ionic equation will be :
\(Hg_2^{2+}(aq)+2NO_3^-(aq)+2Na^+(aq)+2Cl^-(aq)\rightarrow Hg_2Cl_2(s)+2Na^+(aq)+2NO_3^-(aq)\)
HELP PLEASE 1pt
Identify the object that has the greatest gravitational pull.
A.
a tennis ball
B.
the moon
C.
the sun
D.
Earth

Answers
The object with the greatest gravitational pull is the Earth (option D).
What is gravitational pull?Gravitational force is a very long-range, but relatively weak fundamental force of attraction that acts between all particles that have mass. It is believed to be mediated by gravitons.
Newton’s Law of Universal Gravitation states that every particle attracts every other particle in the universe with force directly proportional to the product of the masses and inversely proportional to the square of the distance between them.
The force of gravitational attraction is directly dependent upon the masses of both objects and inversely proportional to the square of the distance that separates their centers.
So as the mass of either object increases, the force of gravitational attraction between them also increases. If the mass of one of the objects is doubled, then the force of gravity between them is doubled. If the mass of one of the objects is tripled, then the force of gravity between them is tripled.
According to this question, Earth has the highest mass among the listed objects, hence, in accordance to the law of universal gravitation, Earth will possess the greatest gravitational pull.
Learn more about gravitational pull at: https://brainly.com/question/13467280
#SPJ1
A student made a sketch of a potential energy diagram to represent an endothermic reaction.
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent. (10 points)

Answers
Answer:
Incorrect
Explanation:
X is the reactants
Y is the products
In an endothermic reaction where energy is absorbed, the enthalpy the products (Y) is always higher than the enthalpy of the reactants (X).
Enthalpy change (∆H) = products (Y) - Reactants (X)
For an endothermic reaction, the ∆H value should be positive.
Enthalpy change
The diagram sketched by the students shows the opposite. The diagram shows that the enthalpy of the products (Y) is lower than the enthalpy of the reactants (X). This will give a negative value of ∆H.
It represents an exothermic reaction rather than endothermic.
Therefore, the student is incorrect.
A common characteristic of killers who choose poison as their weapon. This way they can avoid having to confront their victim me directly.
Answers
Answer:
The killers who choose poison for killing the victim are the one who does not want to confront the victim. When a knife or a bullet is used to kill a person he may struggle and can cause harm to killer also.
Explanation:
Poison is the most easiest way to kill a person without any struggle. The poison can be given to a person in a juice or through an injection. The poison entered in the body of victim will cause his heart to cease gradually and he will not have energy to struggle with the killer to save his life.
can someone please draw 5 lab apparatus
I promise to give brainliest
Answers
Answer:
picture 1 is your answer.
for more details, follow pic 2 and 3



Hydrogen forms _____ bonds with strongly _______ element
Answers
Answer:
Hydrogen bond is formed only by the three highly electronegative elements- fluorine, oxygen and nitrogen.
HELP ME FOR EXTRA POINTSS & brainlest!

Answers
Answer: 1. Endoplasmic Reticulum
Explanation:
it has passageways that carry proteins and other materials from one part of the cell to another
Name the following parts:
A—-
B——
C——

Answers
B—-Neutron
C—-Proton