When this reaction is run , 57.75 g H2O is produced. What is the percent yield for this result?
Answers
The theoretical yield is the amount of product that would be obtained if the reaction proceeded with 100% efficiency.
Once you have the theoretical yield and the actual yield (which is given as 57.75 g of H2O in this case), you can use the following formula to calculate the percent yield:
Percent Yield = (Actual Yield / Theoretical Yield) x 100
In this case, the actual yield is 57.75 g and the theoretical yield is 60.00 g. Therefore, the percent yield is:
Percent yield = (57.75 g / 60.00 g) * 100% = 96.25%
Therefore, the percent yield for this reaction is 96.25%.
To know more about theoretical yield:
https://brainly.com/question/14966377
#SPJ1
Related Questions
HELP PLEASE 1pt
Identify the object that has the greatest gravitational pull.
A.
a tennis ball
B.
the moon
C.
the sun
D.
Earth

Answers
The object with the greatest gravitational pull is the Earth (option D).
What is gravitational pull?Gravitational force is a very long-range, but relatively weak fundamental force of attraction that acts between all particles that have mass. It is believed to be mediated by gravitons.
Newton’s Law of Universal Gravitation states that every particle attracts every other particle in the universe with force directly proportional to the product of the masses and inversely proportional to the square of the distance between them.
The force of gravitational attraction is directly dependent upon the masses of both objects and inversely proportional to the square of the distance that separates their centers.
So as the mass of either object increases, the force of gravitational attraction between them also increases. If the mass of one of the objects is doubled, then the force of gravity between them is doubled. If the mass of one of the objects is tripled, then the force of gravity between them is tripled.
According to this question, Earth has the highest mass among the listed objects, hence, in accordance to the law of universal gravitation, Earth will possess the greatest gravitational pull.
Learn more about gravitational pull at: https://brainly.com/question/13467280
#SPJ1
Suppose that the bond market and the money market both start out in equilibrium, then the Federal Reserve decreases the money supply. The result will be a ______________ in the money market and a _________________ in the bond market, which will push bond prices _________________ and interest rates will ___________________ until a new equilibrium is reached.
Answers
The result will be a decrease in the money market and an increase in the bond market, which will push bond prices down and interest rates will rise until a new equilibrium is reached.
Suppose the Federal Reserve decreases the money supply, this action will lead to a decrease in the money market equilibrium and an increase in the bond market equilibrium. As a result, bond prices will drop, and interest rates will rise until a new equilibrium is established. This shift will occur because as the money supply decreases, the demand for money will increase, leading to an increase in interest rates.
This increase in interest rates will then cause bond prices to drop as the cost of borrowing rises, and investors will demand a higher yield. Ultimately, a new equilibrium will be established where the demand for money equals the supply, and the bond market is in equilibrium with the new interest rate.
More on markets: https://brainly.com/question/32114875
#SPJ11
Why is a hurricane classified as a natural hazard, while air pollution, acid rain, and ozone depletion are not?
A. A hurricane can vary in severity and destructiveness.
B.
A hurricane is a sudden, destructive event that cannot be predicted accurately.
0000
C.
A hurricane causes significant damage across a wide area.
D. A hurricane is a natural process, not the result of human activity.
Answers
Answer: D. A hurricane is a natural process, not the result of human activity.
Explanation: A hurricane is classified as a natural hazard because it is a natural phenomenon that occurs due to specific meteorological conditions, such as high ocean temperatures and specific wind patterns. It is not caused by human activity, unlike air pollution, acid rain, and ozone depletion, which are largely the result of human activities such as industrialization and deforestation. While hurricanes can vary in severity and destructiveness, and can cause significant damage across a wide area, it is ultimately the natural origin of the event that classifies it as a natural hazard.
how many
moles are in 9.01 x 1023 molecules of water. answer should be rounded to three significant figures.
A. 1.50
B. 15.0
C. 0.150
D. None of the above
Answers
Answer:
A. 1.50
Explanation:
9.01 x 1023 molecules* 1 mol/ 6.022×1023 molecules= 1.49618067087 moles
Rounded would be 1.50
2. List and explain the three steps for solving a numeric problem.
Answers
Answer:
what is matter with example
he pH of a 0.11 M solution of chloroacetic acid (CH2ClCOOH) is measured to be 1.91. Use this information to determine a value of Ka for chloroacetic acid.CH2ClCOOH(aq)+H2O(l)⇌CH2ClCOO−(aq)+H3O+(aq)
Answers
The Ka of chloroacetic acid is equal to 2.1 x 10⁻². The Ka for chloroacetic acid can be determined from the measured pH of a 0.11 M solution of chloroacetic acid.
To determine the value of Ka for chloroacetic acid (CH2ClCOOH), we can use the pH of the solution and the initial concentration of the acid. The equation for the dissociation of chloroacetic acid is:
CH2ClCOOH(aq) + H₂O(l) ⇌ CH2ClCOO-(aq) + H₃O+(aq)
At equilibrium, we can assume that x is the concentration of the hydronium ion (H₃O+) and the acetate ion (CH2ClCOO-), which will be equal since the acid is monoprotic. Therefore, the concentration of CH2ClCOO- will also be x. The initial concentration of CH2ClCOOH is 0.11 M.
The equilibrium expression for Ka is given by:
Ka = [CH2ClCOO-][H₃O+]/[CH2ClCOOH]
Substituting the equilibrium concentrations, we have:
Ka = (x)(x)/(0.11 - x)
Given that the pH of the solution is 1.91, we can calculate the concentration of H₃O+ using the relationship:
pH = -log[H₃O+]
1.91 = -log[H₃O+]
[H₃O+] = 10^(-pH)
[H₃O+] = 10^(-1.91)
[H³O+] ≈ 7.94 × 10⁻² M
Since the concentration of H3O+ is equal to x, we can substitute this value into the equilibrium expression:
Ka = (7.94 × 10⁻²)(7.94 × 10⁻²)/(0.11 - 7.94 × 10⁻²)
The Ka of chloroacetic acid is equal to 2.1 x 10⁻².
Know more about chloroacetic acid here
https://brainly.com/question/15049102#
#SPJ11
Name a intensive and extensive property of iron
Answers
Answer: An extensive property is a property that depends on the amount of matter in a sample. Mass and volume are examples of extensive properties. ... Color, temperature, and solubility are examples of intensive properties.
Explanation:
An intensive property of iron is density while an extensive property of iron is mass.
WHAT ARE INTENSIVE AND EXTENSIVE PROPERTIES?
Intensive properties of a substance/element are those properties that do not depend on the amount of matter. Examples of intensive properties are density, boiling point, melting point etc. Extensive properties are those properties that depend on the amount of matter in a substance. Examples are mass, weight, volume etc.Regarding iron as an element, one intensive property it posseses is density while one extensive property is its mass.
Learn more: https://brainly.com/question/3000870?referrer=searchResults
Based on the ideal gas law, what volume of hydrogen gas do you predict would be evolved given the number of moles of zinc and the temperature and pressure in the room during the first part of the experiment? The formula of the ideal gas law is PV=nRT and you can rearrange the equation in order to solve for the volume as follows V= nRT/P (V is the volume, n is the number of moles, R is the gas constant 0.08206 atm*L/mol*K, and T is the temperature in Kelvin (Kelvin =oC + 273.15).3.8 x 10^-3 L0.0922 L200 L22.4 L4.48 L
Answers
We predict that 24.45 L of hydrogen gas would be evolved under these conditions. Option D is correct.
To calculate the volume of hydrogen gas evolved, we need to know the number of moles of hydrogen gas produced, the temperature in Kelvin, the pressure in atm, and the gas constant. Let's assume that the reaction produces 1 mole of hydrogen gas and the temperature and pressure in the room are 25°C (298.15 K) and 1 atm, respectively.
Using the ideal gas law, we can calculate the volume of hydrogen gas evolved as;
V = nRT/P
V = (1 mol) x (0.08206 Latm/molK) x (298.15 K) / (1 atm)
V = 24.45 L
Hence, D. is the correct option.
To know more about hydrogen gas here
https://brainly.com/question/11426882
#SPJ4
--The given question is incomplete, the complete question is
"Based on the ideal gas law, what volume of hydrogen gas do you predict would be evolved given the number of moles of zinc and the temperature and pressure in the room during the first part of the experiment? The formula of the ideal gas law is PV=nRT and you can rearrange the equation in order to solve for the volume as follows V= nRT/P (V is the volume, n is the number of moles, R is the gas constant 0.08206 atm×L/mol×K, and T is the temperature in Kelvin (Kelvin =oC + 273.15). Options: A) 3.8 x 10⁻³ L B) 0.0922 L C) 200 L D) 24.45 L E) 4.48 L."--
Complete the mechanism for the acid‑catalyzed alcoholysis of the epoxide by adding any missing atoms, bonds, charges, nonbonding electrons, and curved arrows.
Answers
The lone pair electron in alcohol undergoes protonation and stabilizes the cation that is present in the oxygen atom. The final product contains both the ether and alcohol functional groups.
The chemical element with the letters O and the atomic number 8 is called oxygen atoms. It belongs to the chalcogen group of the periodic table and is a highly reactive nonmetal that readily forms oxides with most elements as well as with other compounds. Chemical elements, or substances with only one sort of atom, include molecular oxygen. An oxygen atom has eight protons in its nucleus, as indicated by its atomic number of 8 and the official chemical symbol O. The manufacture of steel, plastics, and textiles, brazing, welding, and cutting of steel and other metals, rocket propellant, oxygen therapy, and life support systems in aircraft, submarines, spaceflight, and diving are just a few common uses for oxygen. Since many organisms use molecular oxygen for respiration, molecular oxygen is necessary for life.
Learn more about oxygen atoms here:
https://brainly.com/question/7716347
#SPJ4
Why are engineers called applied scientist?
Answers
Answer:
Applied science is a discipline that is used to apply existing scientific knowledge to develop more practical applications, for example: technology or inventions. In natural science, basic science (or pure science) is used to develop information to explain phenomena in the natural world.
Answer:
• Because engineers apply science and its theories into practical situation
Take an example of;
→ A generator: When creating a generator, electromagnetic induction theorem is applied. [ self and mutual inductance ]
→ A plane: The wings are made in considerance of Bernoulli principle and viscous drag
\(.\)
what is the gram formula mass of NazC03
Answers
Answer:
84,007 g/mol
Explanation:
Molar mass of NazCO3 which is Sodium Bicarbonate (NaHCO₃) is 84,007 g/mol
Calculate the volume of 1. 5x10^-2 naoh that must be added to 500ml og 0. 2m hcl to give a solution that has a ph of 2. 15
Answers
The volume of 1.5 × 10^(-2) M NaOH that must be added to 500 mL of 0.2 M HCl to give a solution that has a pH of 2.15 is 6.67 mL.
To solve this problem, we first need to write the balanced chemical equation for the reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl), which is
NaOH + HCl → NaCl + H2O.
We are given the initial concentration of HCl as 0.2 M. We then use the pH value, which is 2.15, and the relationship between pH and the concentration of hydrogen ions ([H+]) to calculate the concentration of [H+] in the final solution.
Using the equation pH = -log[H+] and [H+] = 10^(-pH),
pH = -log[H+]
[H+] = 10^(-pH)
[H+] = 10^(-2.15) = 7.08 × 10^(-3) M
we find that [H+] = 7.08 × 10^(-3) M.
Next, we need to determine the mole ratio of NaOH to HCl, which we can find from the balanced chemical equation as 1:1. We then calculate the amount of NaOH required to react with all of the HCl in the initial solution, which is 0.1 moles.
moles of HCl = concentration × volume = 0.2 M × 0.5 L = 0.1 moles
moles of NaOH = 0.1 moles
Finally, we need to calculate the volume of 1.5 × 10^(-2) M NaOH required to provide 0.1 moles of NaOH.
moles of NaOH = concentration × volume
volume = moles of NaOH / concentration
volume = 0.1 moles / 1.5 × 10^(-2) M = 6.67 L
For more question on pH click on
https://brainly.com/question/172153
#SPJ11
A Blood Test Indicates The Presence Of A Particular Disease 93% Of The Time When The Disease Is Actually Present. The Same Test Indicates The Presence Of The Disease Given That The Test Indicates The Presence Of The Disease. Give Your Answer In Decimal Form, Rounding To Four Decimal Places.
Answers
Given that a blood test indicates the presence of a particular disease 93% of the time when the disease is actually present, and we want to find the probability of the presence of the disease when the test indicates the presence of the disease.We can solve the problem by using Bayes' theorem. The probability of the disease being present when the test indicates the presence of the disease would be 0.1167 (rounded to four decimal places).
Bayes' theorem states that:
P(A|B) = P(B|A) × P(A) / P(B)
Where;P(A|B) is the probability of A occurring given that B has occurred.
P(B|A) is the probability of B occurring given that A has occurred.
P(A) is the probability of A occurring.
P(B) is the probability of B occurring
.Using the above notation, let;
A = Presence of the disease.
B = The blood test indicates the presence of the disease.
P(A|B) = The probability of the disease being present when the test indicates the presence of the disease.
P(B|A) = The probability of the blood test indicating the presence of the disease when the disease is present.
P(A) = The probability of the disease being present.
P(B) = The probability of the blood test indicating the presence of the disease.
When the test indicates the presence of the disease, the possible outcomes are:
Presence of the disease when the test indicates the presence of the disease.Presence of the disease when the test indicates the absence of the disease.No presence of the disease when the test indicates the presence of the disease.No presence of the disease when the test indicates the absence of the disease.The probability of the blood test indicating the presence of the disease when the disease is present is 93% or 0.93.P(B|A) = 0.93
The probability of the disease being present is not given in the problem statement, but we can assume it to be a small probability. Hence, we will assume P(A) to be 0.01 or 1%.P(A) = 0.01
To find the probability of the blood test indicating the presence of the disease, we can use the law of total probability. The possible outcomes when the test indicates the presence of the disease are presence and absence of the disease, i.e.,P(B) = P(B|A) × P(A) + P(B|not A) × P(not A)P(not A) = 1 - P(A) = 1 - 0.01 = 0.99P(B) = 0.93 × 0.01 + P(B|not A) × 0.99
We know that the blood test indicates the presence of a particular disease 93% of the time when the disease is actually present. Hence, we can assume that the blood test indicates the presence of the disease 7% of the time when the disease is not present.
P(B|not A) = 0.07P(B) = 0.93 × 0.01 + 0.07 × 0.99 = 0.0796
Using Bayes' theorem:
P(A|B) = P(B|A) × P(A) / P(B)P(A|B) = 0.93 × 0.01 / 0.0796 = 0.1166 ≈ 0.1167
Hence, the probability of the disease being present when the test indicates the presence of the disease is 0.1167 (rounded to four decimal places).
Answer: 0.1167
Learn more about probability at https://brainly.com/question/32117953
#SPJ11
The final molarity when adding 125 mL of water to 25.0 mL of a 3.0 M solution of KOH is Blank 1. Round atomic masses to the nearest whole number. Include 2 sig figs total in your answer.
Answer ASAP please thank you
Answers
The final molarity when adding 125 mL of water to 25.0 mL of a 3.0 M solution of KOH is 0.5 M
How do i determine the final molarity of the solution?First, we shall list out the given parameters from the question. Details below:
Initial volume of KOH solution (V₁) = 25 mLInitial molarity of KOH solution (M₁) = 3.0 MVolume of water added = 125 mLFinal volume of KOH solution (V₂) = 25 + 125 = 150 mL Final molarity of KOH solution (M₂) =?The final molarity of KOH solution can be obtained by using the dilution formular as illustrated below:
M₁V₁ = M₂V₂
3 × 25 = M₂ × 150
75 = M₂ × 150
Divide both side by 150
M₂ = 75 / 150
M₂ = 0.5 M
Thus, we can conclude that the final molarity of KOH solution is 0.5 M
Learn more about dilution:
https://brainly.com/question/15022582
#SPJ1
David pushes the wall to the left with his finger. The
wall pushes David to the right. What is the action
force and what is the reaction force?
Answers
Answer:
push or pull
Explanation:
uppose the reaction Ca3(PO4)2 + 3H2SO4 ï‚® 3CaSO4 + 2H3PO4 is carried out starting with 153 g of Ca3(PO4)2 and 87.6 g of H2SO4. How much phosphoric acid will be produced?
Answers
The answer is 58.4 g of H₃PO₄
Given that mass of Ca₃(PO₄)₂ is 153g
mass of H₂SO₄ is 87.6g
We need to calculate the mass of H₃PO₄
So the balanced chemical reaction is
Ca₃(PO₄)₂ + 3H₂SO₄ ⇒ 3CaSO₄ + 2H₃PO₄
Let us calculate the molar mass of the reactants
Ca₃(PO₄)₂ = (3 x 40) + (2 x 31) + (8 x 16)
= 120 + 62 + 128
= 310 g
H₂SO₄ = (1 x 2) + (32 x 1) + (16 x 4)
= 2 + 32 + 64
= 98 g
Now let us calculate the limiting reactant
The Theoretical Yield = Ca₃(PO₄)₂ / H₂SO₄
= 310 / 3(98)
= 1.05
The Experimental yield
Ca₃(PO₄)₂ / H₂SO₄
= 153 / 87.6 = 1.74
Because the observed percentage was more than the predicted proportion, H2SO4 is the limiting reactant.
Let us Calculate the molar mass of H₃PO₄
H₃PO₄ = (1 x 3) + (31 x 1) + (16 x 4)
= 3 + 31 + 64
= 98 g
Now Calculate the mass of H₃PO₄
3(98) g of H₂SO₄ ------------------ 2(98) g of H₃PO₄
87.6 g of H₂SO₄ ------------------ x
x = ( 87.6 x 2 x 98) / (3 x 98)
x = 17169.6 / 294
x = 58.4g of H₃PO₄
Learn more about Theoretical Yield here
https://brainly.com/question/25996347
#SPJ1
Which of the following quantities are required for calculating density? Select all that required.
Volume
Area
Mass
Weight
Answers
Answer:
Mass and Volume
Explanation:
The formula for density is
\(\frac{Mass}{Volume}\)
Which of these pure substances do you think are Elements?
Au (Gold)
C6H12O6 (Sugar)
Fe (Iron)
NaCl (Salt)
O (Oxygen)
Fe2O3 (Rust)
Answers
The others are compounds
how does an atom of nirogen become an ion
Answers
Answer: A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon.
Explanation:
which of the following chemicals provide health benefits and give plant foods their color, aroma, and flavor?
Answers
Plant foods are rich in phytochemicals, which are natural compounds that provide numerous health benefits. These phytochemicals are responsible for the color, aroma, and flavor of plant foods. Some of the important phytochemicals that provide health benefits include flavonoids, carotenoids, and anthocyanins.
Flavonoids are antioxidants that protect the body from damage caused by free radicals. They are found in many plant foods, including berries, citrus fruits, tea, and dark chocolate. Carotenoids are pigments that give plant foods their bright colors, such as red, yellow, and orange. They are converted into vitamin A in the body and have been linked to a lower risk of cancer, heart disease, and age-related eye diseases. Carotenoids are found in fruits and vegetables like carrots, tomatoes, sweet potatoes, and spinach.
Anthocyanins are pigments that give fruits and vegetables their deep red, blue, and purple colors. They are potent antioxidants and have been shown to reduce inflammation, protect against heart disease, and improve cognitive function. Foods that are high in anthocyanins include berries, grapes, red cabbage, and eggplant.
In summary, the phytochemicals flavonoids, carotenoids, and anthocyanins provide health benefits and give plant foods their color, aroma, and flavor. Including a variety of colorful fruits and vegetables in your diet is a great way to ensure that you are getting a range of phytochemicals to support your health.
To know more about Compounds visit :
https://brainly.com/question/14117795
#SPJ11
What change would occur in the mass between the reactants (lead nitrate and potassium iodide) and the products (lead iodide and potassium nitrate)?
Please help me this is coming in my exam. Thank you
Answers
Answer:
Pb(NO
3
)
2
+
2
KI
→
PbI
2
+
2
KNO
3
Explanation:
Volume occupied 3.52x10^32 moluchles
of Mathane (CH4)
1) At STP
Answers
Answer:
volume = 13097674418.528dm³
Explanation:
n = (3.52)*10^32/(6.02)*10^23)
n = (584717607.97)
n = volume /molar volume
molar volume at stp = 22.4dm³
volume= 584717607.97 x 22.4
volume = 13097674418.528dm³
MgCl2 has a theoretical van’t Hoff factor of 3 because it dissociates into three ions when dissolved in water. The measured value of the van’t Hoff factor for MgCl2 is typically no higher than 2.7. Explain why measured values of the van’t Hoff factors for ionic compounds are always lower than the theoretical values.
Answers
Magnesium iodide will dissociate into 1 mole of Mg2+ M g 2 + and 2 moles of I− . This means that it has a Van't Hoff factor of 3.
What is the vant Hoff factor of mgi2?MgCl2 dissociates into three ions when it dissolves in water, giving it a theoretical van't Hoff factor of 3. The van't Hoff factor for MgCl2 is normally tested at a value of no more than 2.7.
The van 't Hoff factor is virtually 1 for the majority of non-electrolytes dissolved in water. The van 't Hoff factor for the majority of ionic compounds dissolved in water is equal to the number of discrete ions in the substance's formula unit.
Only perfect solutions can claim this as ion pairing occasionally happens in solutions. The quantity of ions that develop during the dissociation of an ionic molecule is known as the van't Hoff factor. In the case of a non-ionizing molecule, the van't Hoff factor is 1.
To learn more about van't Hoff factor refer to:
https://brainly.com/question/22047232
#SPJ4
A liquid has a density of 0.70g/ml. Find the mass of the liquid which can be put
into a beaker holding 130mL.
Answers
Answer:
The answer is
91 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density of liquid = 0.70 g/mL
volume = 130 mL
The mass is
mass = 0.7 × 130
We have the final answer as
91 gHope this helps you
Complete the following nuclear equation:

Answers
The nuclear equation shown is completed as follows:
²¹⁴₈₂Pb ---> ⁰₋₁e + ²¹⁴₈₃Bi
The correct option is B.
What is a nuclear equation?A nuclear equation is an equation that represents or shows a nuclear reaction.
particles found nuclear reaction is a reaction in which reaction changes occur in the atomic particles found within the nucleus of a na atom.
There are two types of nuclear reactions;
nuclear fusion - the fusion of the atoms of two sammlerf nuclei to form larger nuclei
nuclear fission - the splitting of the nucleus of a large atom to produce two or smaller atoms.
Learn more about nuclear reactions at: https://brainly.com/question/25387647
#SPJ1
the chemical equation above demonstrates that--question 4 options:the atoms on the reactants side are changed into different atoms on the products sidethe reactants are the same substances as the productsmatter cannot be created or destroyed by a chemical reactionthe number of molecules in the reactants if equal to the number of molecules in the products
Answers
A substance that changes in a chemical reaction and is represented by the reaction arrow on the left side of a chemical equation.
A substance formed in a chemical equation. and written on the right side of the reaction arrow in a chemical equation is referred to as a product. According to the law of conservation of matter, matter cannot be created or destroyed. The number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products in chemical equations. The atoms and molecules that interact in a chemical reaction are referred to as reactants. The atoms and molecules produced by a chemical reaction are referred to as products.
To learn more about chemical equation. please click on below link
https://brainly.com/question/28294176
#SPJ4
provide an explanation that is grounded in polarity and intermolecular forces that better explains why oil is not miscible in vinegar.
Answers
Oil and vinegar cannot be mixed together due to differences in their polarity and intermolecular forces. Oil is a nonpolar substance, while vinegar is a polar substance.
The polarity is defined as the separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. It is the polarity that leads to the formation of hydrogen bonds in a substance. Molecules with hydrogen bonding have strong intermolecular forces and require much energy to separate them. On the other hand, molecules with weak intermolecular forces mix easily with other substances. This explains why oil is not miscible in vinegar.
Oil and vinegar do not mix well because of differences in their polarity and intermolecular forces, oil is nonpolar, while vinegar is polar. Polarity refers to the separation of electric charge in a molecule or its chemical groups leading to an electric dipole moment, with a negatively charged end and a positively charged end. Molecules with strong intermolecular forces have hydrogen bonding and require much energy to separate them, while molecules with weak intermolecular forces mix easily with other substances. Therefore, oil does not mix with vinegar due to differences in their polarity and intermolecular forces.
Learn more about polarity at:
https://brainly.com/question/3248676
#SPJ11
Help me Pleaseeeeeeeee
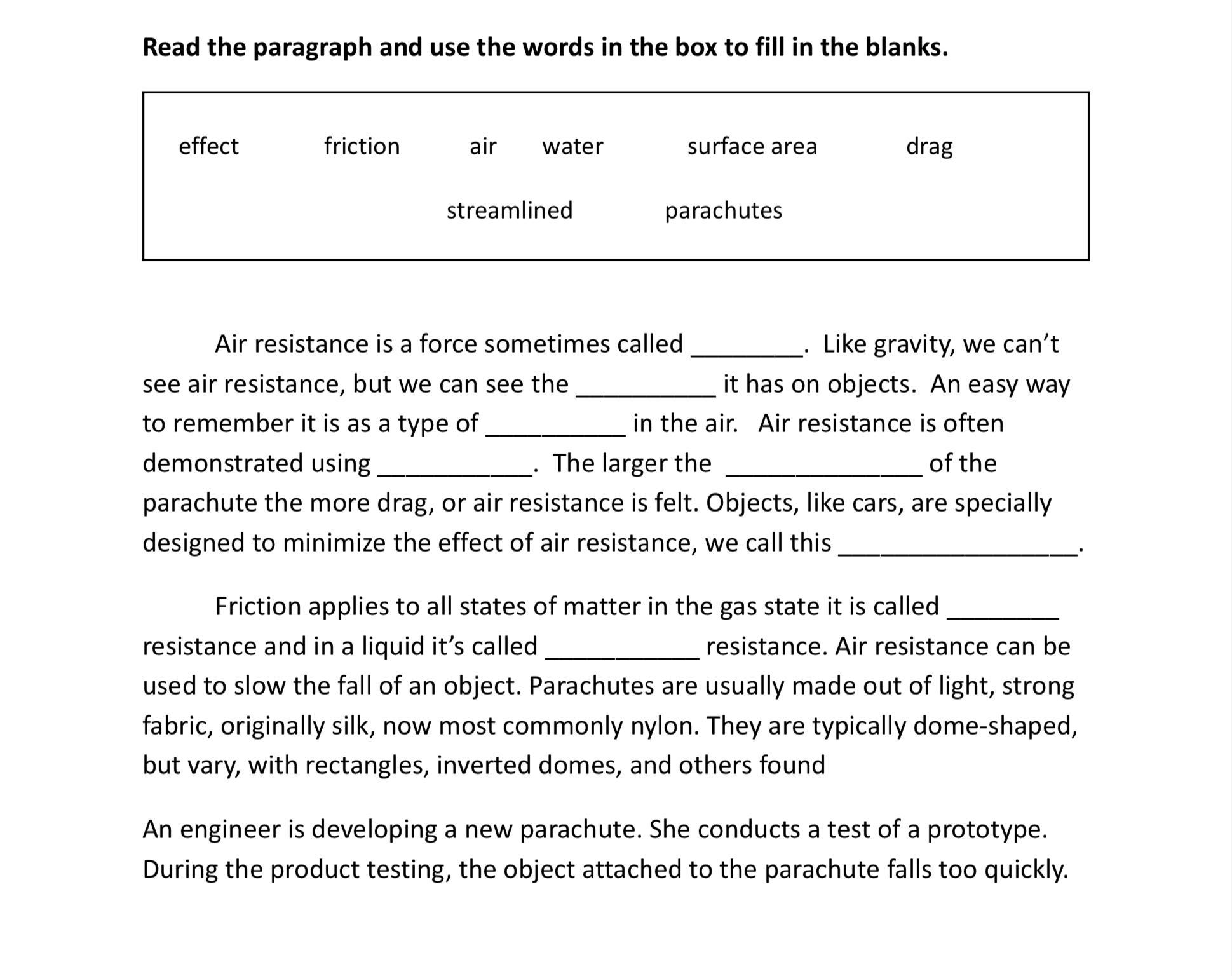
Answers
drag
effect
friction
parachutes
surface area
streamlined
air
water
Students were given samples of simulated Martian soil to observe, describe, and
investigate for any evidence that may indicate that life existed at one time.
Based on the problem being investigated and the data collected, what type of
investigation did the students conduct?
Problem:
What is the composition of the Martian soil sample and what evidence of past life
does it contain, if any?
Composition
Evidence of
Past Life
Data: Observations of Martian Soil Sample
Red soil, sand, tiny translucent rock crystals, bits of brown
rock
Tiny seed-like fossils, what appears to be pieces of fossilized
shells
Answers
Based on the problem being investigated and the data collected, the type of investigation the students conducted is the composition of the Martian soil sample and the evidence of past life on it.
The evidence of past life on Mars is tiny seed-like fossils that appear to be pieces of fossilized shells.
What is the evidence of life on Mars?Mars is the second-smallest planet in the Solar System, only slightly larger than Mercury, and is the fourth planet from the Sun.
Research has sought to find evidence for life on Mars.
Based on the study, tiny seed-like fossils serve as evidence for life on Mars.
Learn more about Mars at: https://brainly.com/question/14838962
#SPJ1
The Lewis dot structure determines the molecular geometry based on boned and unbonded electrons. What is the expected molecular geometry for CO2
Answers
Answer:
The molecular geometry would be linear
Explanation:
Carbon Dioxide has 2 electron domains which would result in a linear geometry, they're not bent because they the lone pairs of electrons are pretty far from each other
This should be a diagram of their structure (Sorry for the terrible drawing skills was in a hurry)
