when a β- particle is emitted from an unstable nucleus, the atomic number of the nucleus:
increases by 1.
increases by 2. decreases by 1.
decreases by 2. does not change.
Answers
When a β- particle is emitted from an unstable nucleus, the atomic number of the nucleus decreases by 1. This is because β- particles are actually electrons that are emitted from the nucleus during beta decay, which occurs when a neutron is converted into a proton and an electron.
The electron is then ejected from the nucleus as a β- particle, while the proton remains in the nucleus. Since the atomic number of an element is determined by the number of protons in its nucleus, the emission of a β- particle results in a decrease in the atomic number. This process can also result in the creation of a new element, as the conversion of a neutron into a proton can change the identity of the element.
When a β- particle is emitted from an unstable nucleus, the atomic number of the nucleus increases by 1. This process occurs because a neutron inside the nucleus transforms into a proton, which results in the release of a β- particle (an electron) and a small, neutral particle called an antineutrino. The increase in the number of protons causes the atomic number to rise by 1.
For more information on β- particle visit:
brainly.com/question/13162951
#SPJ11
Related Questions
Which radioisotope is used to treat thyroid disorders?.
Answers
Answer:
I-123 and I-131
Explanation:
Radioactive iodine takes advantage of the fact that thyroid cells and thyroid cancer cells absorb iodine; therefore, it has been used to diagnose or treat various thyroid disorders. Iodine is made into two radioactive isotopes, I-123 and I-131, that are commonly used in patients with thyroid disease.
Mike Trout of the Los Angeles Angels has a contract worth $426,500,000. If you earn $100,000 per year, how many years would it take for you to earn what Mike Trout will earn from his contract?
Answers
Answer:
The number of years it would take you to earn what Mike Trout will earn on his contract is 4,265
Explanation:
Mike Trout of the Los Angeles Angels has a contract worth $426,500,000
On the other side, you earn $100,000 per year.
To calculate the number of years it would take for you to earn what Mike Trout will earn from his contract, you must divide the amount Mike earned by the amount you earned during a year:
$426,500,000 ÷ $100,000 per year= 4,265
The number of years it would take you to earn what Mike Trout will earn on his contract is 4,265
What is the shortest day of the year known as? A.vernal B.equinox C.winter D.solstice summer solstice E.autumnal equinox
Answers
Answer:
summer solstice
Explanation:
on june solstice the northen hemisphere leans more toward the sun giving us longer days and more strong sunlight and It's the opposite in the Southern Hemisphere where june marks the start of winter thus its the shortest day of the year
hc and co are high and co2 and o2 are low. this could be caused by a
Answers
HC and CO are high and CO₂ and O₂ are low. This could be caused by a rich mixture.
A) rich mixture
If HC (hydrocarbons) and CO (carbon monoxide) levels are high, while CO₂ (carbon dioxide) and O₂ (oxygen) levels are low, it suggests a condition known as a "rich mixture" in the combustion process. A rich mixture refers to an air-fuel mixture in which there is an excess of fuel compared to the amount of air required for complete combustion.
When the fuel-air mixture is rich, it means that there is more fuel available relative to the available oxygen for combustion. This imbalance can occur due to several reasons, such as:
1. Incorrect fuel-to-air ratio: The air-fuel mixture may be adjusted incorrectly, with too much fuel being supplied relative to the amount of air. This can occur due to a malfunctioning fuel injection system.
2. Malfunctioning sensors: The sensors responsible for measuring the oxygen and fuel levels in the exhaust gases, such as the oxygen sensor or air-fuel ratio sensor, may be faulty or contaminated. This can result in inaccurate readings and improper adjustment of the fuel mixture.
3. Clogged air intake or fuel injectors: If the air intake or fuel injectors are clogged, it can disrupt the proper mixing of fuel and air, leading to a rich mixture.
The consequences of a rich mixture include:
High HC levels: A rich mixture results in incomplete combustion, leading to unburned hydrocarbon molecules being released into the exhaust gases. This increases the HC levels.
High CO levels: In a rich mixture, there is an excess of fuel. As a result, some of the fuel does not undergo complete combustion and is converted into carbon monoxide (CO). This leads to elevated CO levels.
Low CO₂ levels: Since there is incomplete combustion in a rich mixture, the amount of carbon dioxide (CO₂) produced is reduced.
Low O₂ levels: A rich mixture consumes most of the available oxygen for combustion, resulting in lower levels of oxygen (O₂) in the exhaust gases.
To know more about rich mixture here
https://brainly.com/question/32482229
#SPJ4
The complete question is:
HC and CO are high and CO₂ and O₂ are low. This could be caused by a ____?
A) rich mixture
B) lean mixture
C) defective ignition component
D) clogged EGR passage
which pollutant are you more likely to encounter in dangerous concentrations indoors rather than outdoors?
Carbon Monoxide
Answers
Carbon Monoxide is a gas that is colorless and odorless. It is generated as a byproduct of burning gas, wood, charcoal, and other organic fuels. Carbon Monoxide is a dangerous pollutant that can be found indoors rather than outdoors in dangerous concentrations.
Carbon Monoxide is a gas that is colorless and odorless. It is generated as a byproduct of burning gas, wood, charcoal, and other organic fuels. Carbon Monoxide is a dangerous pollutant that can be found indoors rather than outdoors in dangerous concentrations. It is critical to keep in mind that Carbon Monoxide concentrations are highest in enclosed spaces. It is a pollutant that is more likely to be found indoors than outside. Carbon Monoxide can be lethal if inhaled in sufficient concentrations.
At lower concentrations, it can cause headaches, nausea, and dizziness. Carbon Monoxide detectors are available to warn you if it is present in the air. Carbon Monoxide is a pollutant that we should be aware of and take precautions against to keep ourselves and our family safe. In conclusion, Carbon Monoxide is a harmful indoor pollutant that should be monitored frequently with alarms, especially in spaces that are not adequately ventilated, and with fuel-burning devices, to avoid dangerous levels that may lead to life-threatening emergencies.
To know more about pollutant visit:
https://brainly.com/question/29594757
#SPJ11
What is the formula for Copper (1) Oxide

Answers
Answer:
Cu₂O
Explanation:
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu₂O. It is one of the principal oxides of copper, the other being or copper (II) oxide or cupric oxide. This red-coloured solid is a component of some antifouling paints.
The following gases were bubbled through water, which of the gases is most likely to dissolve and why?
a. CO2
b. CH4
C.PH
d. N2
Answers
Solubility refers to the ability of substances to dissolve in a solvent. Of the gases listed here, that which would most likely dissolve in water is;
a. CO2Carbon dioxide is a gas that has a very high solubility in water. When bubbled through water, 99% of the gas dissolves while 1% of the gas comes off as carbonic acid.
The solubility of carbon dioxide increases with an increase in pressure and decreases with an increase in temperature.
Therefore, option A is correct.
Learn more here:
https://brainly.com/question/24332155
Lab: Types of Reactions Analysis and conclusion
Answers
Generally, There are several types of chemical reactions, including synthesis, decomposition, single replacement, double replacement, and combustion.
In a single replacement reaction, an element is replaced by another element in a compound.In a double replacement reaction, the ions of two compounds switch places to form two new compounds.In a combustion reaction, a hydrocarbon reacts with oxygen to produce carbon dioxide and water.In conclusion, it is important to understand the different types of chemical reactions as they can have different outcomes and uses in various fields such as chemistry, biology and material science.
Read more about Types of Reactions Analysis and conclusion
https://brainly.com/question/2506879
#SPJ1
NaHCO3+HCI--->NaCI+H2O+CO2
Percent yield:93.4%
how would the percent yield be affected if some sodium hydrogen carbonate is left unreacted? explain
Answers
Answer:
Explanation:
percent yield is ratio of actual yield or experimental yield divided by theoretical yield multiplied by 100 .
percent yield of 93.4 % means , the actual yield is 93.4 % what was expected from the reaction on the basis of given chemical reaction .
If in the experimental process , some sodium hydrogen carbonate is left unreacted due to absence of reactant HCl which is also required to obtain product , the percent yield will be increased if the required HCl is also provided .
Hence the percent yield will be increased if required HCl is made available .
change word equation into chemical equation.
a Calcium carbonate -----> Calcium oxide + Carbon dioxide
b Zinc + Sulphuric acid -----> Zinc sulphate + Hydrogen
Answers
Answer:
Ca(CO3) --> CaO + CO2
Zn + H2SO4 --> ZnSO4 + 2H2
Explanation:
^^^
I WIl GIVE YOU BRAINLIEST

Answers
Answer:
"A" i'm pretty sure
Hope this helps
EASY 100 POINTS, WILL MARK BRAINLEST, 2 QUESTIONS, SHOW YOUR WORK OR NO POINTS

Answers
Answer:
Explanation:
1. Please provide the enthalpy info - I will work on it with the info
2.
i) Reaction a should be modified to match the number of S in equation:
2S + 2O2 -> 2SO2 deltaH = -370kJ
ii) Reaction b should be written reversely to match the reactants of SO2:
2SO2 + O2 -> 2SO3 deltaH = 256kJ
iii) Adding the equations together:
2S + 3O2 -> 2SO3
iv) Enthalpy of the combined reaction = -370+256 = -114kJ
It is negative so the reaction is exothermic.
Answer:
Explanation:
1 c. enthalpy change = enthalphies of formation of all products - enthalphies of formation of all reactants = -526.3 kcal/mol
1 d. C3H8(g) + 5 O2 (g) → 3 CO2 (g) + 4 H2O (l) ΔH = -526.3 kcal/mol
Calcium carbonate reacts with lithium metal to result in a single replacement reaction.
How many grams of each product are formed from 25.0 grams of lithium metal?
Answers
133.0 grams and 72.0 grams of Li2CO3 and Ca would be formed respectively.
From the equation of the reaction:
\(2 Li + CaCO_3 ---> Li_2CO_3 + Ca\)
The mole ratio of Li to Li2CO3 is 2:1 while that of Li to Ca is also 2:1.
Mole of 25.0 grams of Li = mass/molar mass
= 25/6.94
= 3.6 moles
Equivalent of mole of Li2CO3 = 3.6/2
= 1.8 moles
Amount of Li2CO3 formed = mole x molar mass= 1.8 x 73.89
= 133 grams
Equivalent mole of Ca = 3.6/2
= 1.8 moles
Amount of Ca formed = 1.8 x 40= 72 grams
More on stoichiometric calculations can be found here: https://brainly.com/question/2563006?referrer=searchResults
Two students are having a tug of war. The flag on the rope begins at point Y. team A pulls to the left with force of 120 N. Team B pulls to the right with force of 160 N. Based on these forces, which letter shows the likely movement of the flag?
Choices:
A: W
B: X
C: Y
D: Z
(Please help!!) 20 points

Answers
Answer:
the answer is Z b/c wehave to forces and they have different direction. then search the sum of the forces. thus we gain 40 N to right.
Predict the organic products from reaction of 2-pentyne with H2O, H2SO4, HgSO4.
Question:
Predict the organic products from reaction of 2-pentyne with H2O, H2SO4, HgSO4.
Hydration of alkynes:
Alkynes undergo acid catalyzed hydration in the presence of mercuric sulfate to give ketones. Symmetric alkynes will only give one ketone product. Internal asymmetric alkynes will give two alkyne products.
Answers
The reaction of 2-pentyne (an internal asymmetric alkyne) with H2O, H2SO4, and HgSO4 would undergo acid-catalyzed hydration to yield two ketone products. The predicted organic product is
2- Pentyne + H2O/H2SO4/HgSO4 → 2-Pentanone + 3-methyl-2-pentanone
The first ketone product is 2- pentanone, which is formed by the addition of a water molecule across the triple bond, resulting in the formation of a ketone group.
The second ketone product is 3-methyl-2-pentanone, which is formed by the addition of a water molecule across the triple bond, resulting in the formation of a ketone group. In this case, due to the internal asymmetry of 2-pentyne, the addition can occur on either side of the triple bond, leading to the formation of two isomeric ketones. The given name represents one of the possible isomers.It's important to note that the reaction is acid-catalyzed, with H2SO4 serving as the acid catalyst and HgSO4 playing a role in the overall reaction. The acid catalyst facilitates the addition of water across the triple bond, while HgSO4 helps in activating the triple bond for hydration.
Learn more about Ketone here, https://brainly.com/question/23849260
#SPJ11
as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
someone please help me with this????

Answers
Answer:
D po ayan po ang sagot ko salamat po
s this a speed, vebcty,
or acceleraton
Abogter
canoeng at 27
mmn
Answers
Answer:
the answer is b
Explanation:
i just answer he question and got it right 2022
The density of a gas was found to be 19.1 g/L at 2.25 atm and 19.3°C. What is the molar mass of
the gas?
Answers
Answer:
Explanation:
Recall that the density of a gas is its mass to volume ratio,
ρ
=
m
V
. Therefore, if we can determine the mass of some volume of a gas, we will get its density. The density of an unknown gas can used to determine its molar mass and thereby assist in its identification. The ideal gas law, PV = nRT, provides us with a means of deriving such a mathematical formula to relate the density of a gas to its volume
The relative number of atoms of a compound can be calculated
by dividing the percentage of an element by the:
Answers
Answer:
Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.
Answer:
Obtain the relative numbers of atoms of each element in the compound by dividing the number of moles of each element in the 100 g sample by the number of moles of the element present in the smallest amount.
Baking cookies are what type
of change?
Chemical physical or thermal?
Answers
Answer:
chemical
Explanation:
which groups have elements with more than one positive oxidation state?
Answers
Answer:
(Ar, Xe, Rn) is the answer
According to the electronic configuration groups three to twelve have elements with more than one positive oxidation state.
What is electronic configuration?
Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/13497372
#SPJ6
Most strong electrolyte solutions are not ideal so that the measured value of the van't Hoff factor i is _____ than expected from the formula of the compound. This is because ions in solution are not totally isolated from each other, but remain clustered together to form an ionic atmosphere. This _____ the effective concentration of particles and therefore the measured value of i.
Answers
Answer: Van't Hoff factor, i is less than expected from the formula of the compound and this decreases the effective concentration of particles.
Explanation:
Strong electrolytes are defined as the solutions which completely dissociate into their ions.
But, most of the strong electrolytes do not dissociate completely (act ideally) but remain partially undissociated into the ions. Thus, giving a lesser number of ions in the solution than expected.
This means that the Van't Hoff factor, i which denotes the number of ions is less than expected.
As fewer ions are released in the solution, the concentration of the solution is expected to decrease.
Hence, van't Hoff factor, i is less than expected from the formula of the compound and this decreases the effective concentration of particles.
Ethylene glycol (C2H6O2) is used as an antifreeze in cars. If 250 g of ethylene glycol is added to 3.00 kg of water, what is the molality? Calculate how much the freezing point of water will be lowered. The freezing-point depression constant for water is Kf = –1.86°C/m. Show your work.
Answers
Answer:
2,909 M
Explanation:
molair mass is of.ethylene is 26,04 g/mol
first you need to calculate how much mL 3 kg is. You can do this by using the density of ethylene: 1,1 g/mL.
3000 g x 1.1 = 3300 mL = 3,3 L
Next you need to calculate the amount of moles:
250 g / 26,04 g/mol = 9,60 mol
Now you can calculate the molarity:
9,6/3.3 = 2,909 M
I don't know the answer for the second question. I'm sorry.
Use the particle theory to explain why 10 mL
of liquid cannot
fill a 20 mL container.
Answers
Answer:
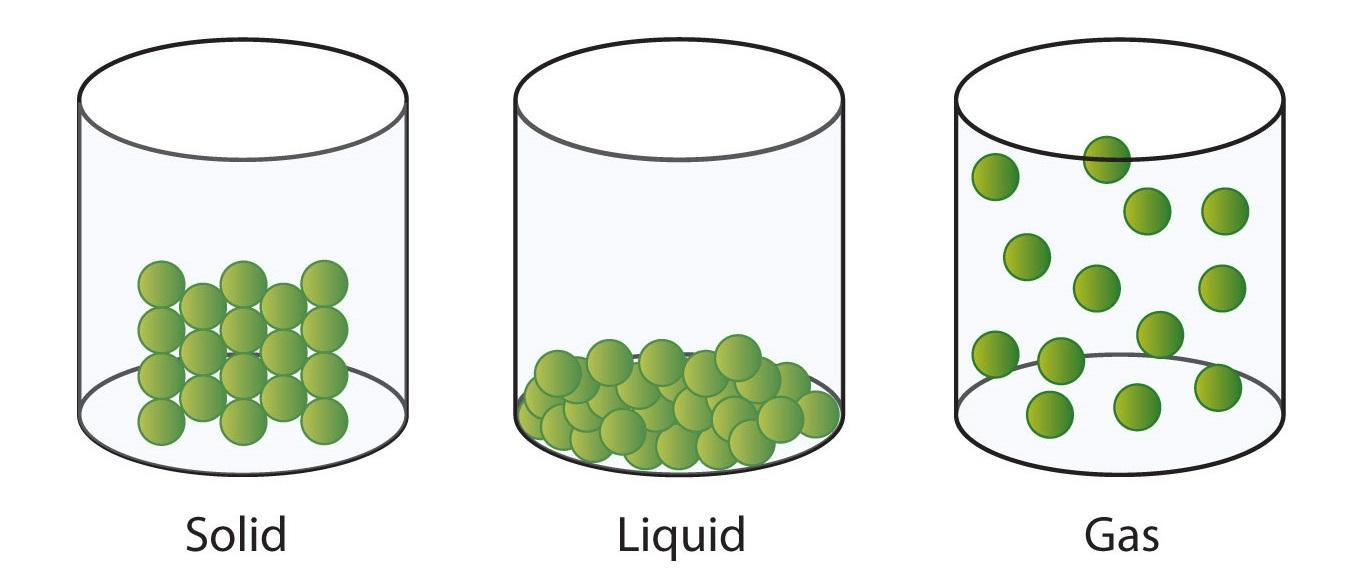
The particle theory is the belief that everything in our solar system and beyond is made of very small matter called atoms. The 10 mL is a matter in our solar system and even though we cannot see, there are millions- if not billions of smaller particles that make up the liquid. If you view the picture below, you can see that the particles in a liquid are close to one another, particles in a gas are far apart, and a particle in a solid is tightly pushed together. This gives them their distinctive shape. Since this is a liquid, this means that the particles are close together, but not very close. The particles glide over one another. If you want to have more space between the particles and expand the size of the liquid you can boil the water, however boiling the water turns it into gas and causing the liquid to evaporate. Freezing the liquid would cause the particles to be closer packed together, making a solid. The amount of liquid you have can not change. There is still 10 mL even when the liquid is frozen or when the liquid boils into vapor in the air. Therefore using particle theory, we can know that a shape can only expand or shrink when changing states of matter.
I hope this helped & Good Luck <3 !!
84 pt =_____ qt
5.0 gal =_____ L
Answers
Answer:
84 pt =__50.4399___ qt
5.0 gal =___18.9271__ L
Explanation:
Have A Wonderful Day!!
Answer: 84 pints = 42 quarts
5.9 gallons = 1.89271
Explanation:
Identify each category of substance as soluble or insoluble in water.Most carbonate and phosphate salts blankMost halide (Br-, Cl-, and I-) salts blankMost silver salts blanksalts of group 1 elements blankMost nitrate salts blank
Answers
The majority of carbonate and phosphate salts are water insoluble. The majority of halide salts (Br-, Cl-, and I-) are water soluble. The majority of silver salts are water insoluble. Group 1 element salts are water soluble. The majority of nitrate salts are water soluble.
Why is CO3 called carbonate?Carbonate is the term given to the material with the chemical formula CO3. Carbonate has an electric charge of 2 and is composed of 1 carbon atom and 3 oxygen atoms. Due to its negative charge, carbonate contains 2 more electrons than protons in each of its individual ions. The organic molecule with the carbonate group C(O-)2, known as a carbonate ester, may also be referred to as a carbonate.
Is carbonate a base or acid?A moderately powerful base is carbonates. Since the carbonate anion can take an ion of hydrogen from water, aqueous solutions are basic. CO32 + H2O -> HCO3 + OH Gaseous carbon dioxide, water, and metal salts are created when carbonates and acids interact. Infusing carbon dioxide gas under pressure into water results in carbonated water. This creates sparkling water, club soda, soda water, seltzer water, and fizzy water, which are all terms for the frothy beverage that is the result. Unless it's seltzer water, salt is typically added to carbonated fluids to enhance flavor.
To know more about Carbonate visit:
https://brainly.com/question/29167877
#SPJ4
write the net ionic equation for the reaction of silver chloride with ammonia
Answers
The net ionic equation for the reaction of silver chloride (AgCl) with ammonia (NH₃) can be written as follows:
AgCl(s) + 2NH₃(aq) → Ag(NH₃)₂+(aq) + Cl⁻(aq)
An ionic equation is a chemical equation in which the formulas of dissolved aqueous solutions are written as individual ions. While this form more accurately represents the mix of ions in solution, the presence of so many individual ions can make it harder to visually determine what is occurring in the reaction.
The net ionic equation for the reaction of silver chloride (AgCl) with ammonia (NH₃) can be written as follows:
AgCl(s) + 2NH₃(aq) → Ag(NH₃)₂+(aq) + Cl⁻(aq)
In this reaction, the silver chloride solid reacts with ammonia to form a complex ion called silver ammine complex, Ag(NH₃)²⁺, which is soluble in water. The chloride ion Cl⁻ from silver chloride remains unchanged and remains in the solution as an aqueous ion.
To know more about ionic equation:
https://brainly.com/question/18635091
#SPJ4
How many molecules of methane gas (CH4) exists in a container at STP that is a total of 2. 5 liters?
Answers
There are 6.35 × 10²² CH₄ molecules in the container at STP that is a total of 2.5 liters. To determine the number of molecules of methane gas (CH4) that exists in a container at STP that is a total of 2.5 liters, we first need to know the STP (Standard Temperature and Pressure) values.
These values are 0°C (273.15 K) and 1 atm pressure (101.3 kPa).
So the given parameters in the question are as follows:
Volume = 2.5 Liters
Temperature (T) = 0°C or 273.15 K
Pressure (P) = 1 atm or 101.3 kPa
We can now use the Ideal Gas Law to determine the number of molecules of methane gas that exist in the container at STP.
Ideal Gas Law PV=nRT
where, P = pressure
V = volume
T = temperature
R = universal gas constant
n = number of moles of gas
R = 0.0821 Latm/mol K
The equation can be rearranged as
n = (PV)/(RT)
Where:
n = number of moles of gas
P = pressure
V = volume
T = temperature
R = Universal Gas Constant
Let's calculate the number of moles of methane gas (CH4) that exists in the container at STP:
(P = 1 atm, V = 2.5 L, R = 0.0821 L atm/mol K, T = 273.15 K)n
= (1 atm * 2.5 L)/(0.0821 L atm/mol K * 273.15 K)n
= 0.1056 mol
So, the number of moles of methane gas (CH4) that exists in the container at STP is 0.1056 mol.
Now, we can use Avogadro's number to determine the number of molecules of methane gas (CH4) that exists in the container at STP.1 mol of gas contains 6.022 x 10^23 molecules
So,0.1056 mol of gas will contain
0.1056 mol × 6.022 × 10²³ mol⁻¹
= 6.35 × 10²² CH₄ molecules
To learn more about Standard Temperature and Pressure refer:-
https://brainly.com/question/29129606
#SPJ11
An Ionic bond forms between
A. Two electronegative atoms
B. A cation and an anion
C. Water Molecules
D. Polar Molecules
E. Nonpolar Molecules
Answers
The cation and anion then attract one another through the Coulomb force to form an ionic bond. Each ionic bond is formed between two oppositely charged ions, such as a sodium ion and a chloride ion. Ionic bonding typically occurs between a metal and a nonmetal. Therefore, option B is the correct answer.
Ionic bond forms between a cation and an anion. Ionic bonds occur when a cation (positive ion) interacts with an anion (negative ion) through the Coulomb force. This electrostatic interaction is so powerful that the two ions become inseparable.The correct option is B. A cation and an anionIonic bonding is a type of chemical bonding in which ions with opposite charges are held together by electrostatic attraction. Ionic bonding takes place when one atom completely removes an electron from another, creating ions. When this occurs, the atom that has lost the electron becomes a cation, and the atom that has gained the electron becomes an anion. The cation and anion then attract one another through the Coulomb force to form an ionic bond. Each ionic bond is formed between two oppositely charged ions, such as a sodium ion and a chloride ion. Ionic bonding typically occurs between a metal and a nonmetal. Therefore, option B is the correct answer.
To know more about oppositely visit:
https://brainly.com/question/29134649
#SPJ11