Answers
If we add one or two hydrogen ions to a polyatomic ion that has a 3-charge, as the phosphate ion (PO₄3-), it will still be a polyatomic ion. (Three H+ would entirely cancel out the 3-charge, turning it into a neutral molecule and removing it from the category of polyatomic ions.
Why does carbonate have a negative 2 charge?As a result, the carbonate ion has 2 more electrons than protons due to its negative charge. The doubly bonded oxygen in the carbonate ion is neutral, whereas each single bonded oxygen has a negative charge. This is the cause of the total charge of "-2," then.
An essential component of the atmosphere of stars like the Sun is the hydrogen anion.
learn more about carbonate ion
https://brainly.com/question/28770987
#SPJ1
Related Questions
Chloride ion concentration in the fluid surrounding human cells is approximately 25 mM (millimolar). How many grams of NaCl must be dissolved in 1.00 L of solution to make a 25 mM NaCl(aq) solution?
Answers
1.461 grams of NaCl must be dissolved in 1 liter solution to make 25 mM of NaCl solution according to molar concentration.
What is molar concentration?
Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.
Millimolar concentration is also calculated by the same formula just the difference in units is present. According to the data given and substitution in the formula,
mass= 25×58.44×1=1461 mg or 1.461 g
Thus, 1.461 g of sodium chloride is required to make 25 mM solution.
Learn more about molar concentration,here:
https://brainly.com/question/21841645
#SPJ2
12.00 moles of chlorine
Answers
840 moles of chlorine in 12 moles.
What in chemistry is a mole?
The unit of measurement used by chemists known as a mole is very significant. Similar to how having a dozen eggs says you have twelve eggs, having a mole of something indicates that you have 602,214,076,000,000,000,000,000 of that particular thing.
When measuring very small objects like atoms, molecules, or other particles, chemists must use moles. 6.02214 x 1023 particles make up a mole, which is equal to (10) of material. 6.02214 1023 particles make up one mole.
1 mole of chlorine weighs 70 grams.
12.00 moles of chlorine = 12 * 70
= 840
Learn more about mole
brainly.com/question/26416088
#SPJ1
The complete question is -
How many grams are in 12.00 moles of chlorine (gas)?
state the types of matter
Answers
Answer:
they're
Explanation:
solid, liquid and gas
A 0.682 g sample of a weak monoprotic acid, HA was dissolved in sufficient water to make 50.0 mL of solution and was titrated with a 0.135 molar NaOH solution. After the addition of 10.6 milliliters of base, a pH of 5.65 was recorded. The equivalence point was reached after the addition of 27.4 milliliters of the 0.135 molar NaOH.
a. Calculate the number of moles of acid in the original sample.
b. Calculate the molar mass of the acid HA.
c. Calculate the [H3O+] at pH = 5.65
d. Calculate the number of moles of unreacted HA remaining in solution when the pH was 5.65.
e. Calculate the value of the ionization constant, Ka, of the acid HA.
f. Calculate the value of the ionization constant, Kb, and explain how you would use it to determine the pH of a solution of a known mass of the sodium salt (Na)(A) dissolved in a known volume of water.
Answers
How to convert butane to 2 butano?
Answers
Answer:
Explanation: Butane can be converted to 2-butano through a process called isomerization. Isomerization is a chemical reaction in which a compound is converted into one of its structural isomers, which are molecules with the same chemical formula but different arrangement of atoms.
To convert butane to 2-butano, one common approach is to use a catalyst such as a metal or metal oxide to facilitate the rearrangement of the atoms in the molecule. This process typically involves heating the butane to a high temperature (around 200-300°C) in the presence of the catalyst, which promotes the isomerization reaction. Other conditions such as pressure and the presence of a solvent may also be used to control the reaction.
It's important to note that this process typically has a low yield, meaning that only a small fraction of the starting butane is converted to 2-butano. As a result, additional purification steps may be needed to separate the 2-butano from the other products of the reaction.
How many moles of aluminum ions al3+ are present in 0.42 mol of al2so43
Answers
There are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
To determine the number of moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3, we need to consider the stoichiometry of the compound.
The formula of aluminum sulfate (Al2(SO4)3) indicates that for every 1 mole of the compound, there are 2 moles of aluminum ions (Al3+). This means that the mole ratio of Al3+ to Al2(SO4)3 is 2:1.
Given that we have 0.42 mol of Al2(SO4)3, we can calculate the moles of Al3+ as follows:
Moles of Al3+ = 0.42 mol Al2(SO4)3 x (2 mol Al3+ / 1 mol Al2(SO4)3)
Moles of Al3+ = 0.42 mol Al2(SO4)3 x 2
Moles of Al3+ = 0.84 mol Al3+
Therefore, there are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
It's important to note that the stoichiometry of the compound determines the mole ratio between the different species involved in the chemical formula. In this case, the 2:1 ratio of Al3+ to Al2(SO4)3 allows us to determine the number of moles of Al3+ based on the given amount of Al2(SO4)3.
For more such question on aluminum visit:
https://brainly.com/question/30451292
#SPJ8
What molecule represents this structure
A)NH4
B)NH3
C)NH4+
D)NH3+

Answers
Explanation:
As I think Option C is correct i.e. NH4+.
We have been thinking about two claims that are possible explanations for how the channel on Mars was formed. We have considered evidence from images, as well as from the Flowing Water Model and the Flowing Lava Model.
1. Select the claim you think is best supported by the evidence you have seen so far.
Responses
Claim 1: Flowing water formed the channel on Mars.
Claim 1: Flowing water formed the channel on Mars.
Claim 2: Flowing lava formed the channel on Mars.
Claim 2: Flowing lava formed the channel on Mars.
2. Why do you think the claim you selected is best supported by the evidence?
Answers
The claim that can be supported is that the channels in mars are made by the flow of lave and not by water. Thus, claim 2 is supportive.
What is channels in mars?According to the new studies of the surface features of mars there are some channels found through the plates of mars. At least one of the meandering channels on the surface of Mars can possibly be explained by the fact that flowing lava can create or carve patterns that resemble the riverbeds and canyons cut by water.
NASA delivered this talk on these findings on March 4, 2010, at the 41st Lunar and Planetary Science Conference at NASA's Goddard Space Flight Center in Greenbelt, Maryland.
The possibility of discovering life on Mars is supposed to be affected by the question of whether the channels on Mars were produced by lava or by water.
To find more mars, refer here:
https://brainly.com/question/29186894
#SPJ1
once water reach its boiling point what does you notice about its temperature??
Answers
How many pounds in 16.1lbmol of pure HCl
Answers
Answer:
586.846 lbs
Explanation:
the molar mass of HCl is 1 + 35.45 = 36.45
this means you can do 16.1 lbmol * 36.45 lb/lbmol = 586.845
What type of compound is represented by the graph at right? A. strong base B. strong acid C. weak base D. weak acid

Answers
The type of compound represented by the graph at right is a strong acid (option B).
What is a strong acid?An acid is generally any compound capable of dissociating into its respective constituent ions when in an aqueous solution.
An acid is categorised as strong or weak depending on whether it can dissociate completely or partially. A strong acid dissociates completely in water.
According to this question, HA, when added to water, dissociates into H+ and A- ions, hence, is a strong acid.
Learn more about strong acid at: https://brainly.com/question/29769012
#SPJ1
Helium is located in group 8A but does not have eight valence electrons. Why is it located in 8A, not 2A?
Answers
Answer:
its outermost shell is completely full making it extremely stable.
Explanation:
It only has two electrons in its outer shell so its valence electron configuration is 1s2. Even though it only has two electrons, it is grouped with elements that have eight valence electrons. Helium is still happy because its outermost shell is completely full making it extremely stable.
Convert 0.00000000045 to scientific notation.
Answers
Answer:
4.5 multiplied by 10, to the -10th power.
\((4.5 \times 10 { - }^{10} )\)
What happens when two cars converge
Answers
determine the rate law and the rate law constant for the following reaction: bro3 5br 6h -> 3br2 3h2o
Answers
The rate law and the rate law constant for the following reaction: bro3 5br 6h -> 3br2 3h2o is 1.5 × 10-3 M/S.
This means that the molecularity and order of the reaction with regard to [Br] are 5 and 1, respectively. The rate of bromine emergence is related to the rate of bromide ion evaporation in the reaction "BrO 3(-)(aq) + 5Br(-) + 6H(+) rarr 3Br 2 + 3H 2O." The reaction rate for the reaction A->B is increased by 8 times when the concentration of both reactants is doubled, while the reaction rate is only doubled when the concentration of B is doubled. If there are solely saturated chemicals present, it turns reddish-brown. By gradually adding bromine solution until the first reddish-brown hue appears, you may gauge how unsaturated a compound is. The unsaturation increases as more bromine solution is needed.
Learn more about rate law from here:
https://brainly.com/question/30379408
#SPJ4
Is carbon an organic compound?
A.
Yes – it contains carbon
B.
Yes – all compounds are organic
C.
The question can't be answered with the information given.
D.
No – it is not a compound: it's an element.
Answers
carbon is an elements ,The compounds of carbon are called an organic compounds.
Carbon have tendency to catenation. catenation means forming bond with another carbon atom and form a long chain of carbon. This is the reason for the presence variety of number of organic compounds. The atomic size of carbon is small and have the valency of four, forms covalent bond. Carbon is strong and stable. Generally carbon containing compound are organic compounds. Generally all the Organic compounds contain carbon and hydrogen called as hydrocarbon.
Thus, carbon is an elements ,The compounds of carbon are called an organic compounds.
To learn more about Organic compound here
https://brainly.com/question/4059093
#SPJ1
A sample of gas occupies 7.80 liters at 425°C? What will be the volume of the gas at 35°C if the pressure does not change?
Answers
Answer:
How do amoeba respire.
how do plants respire.
Complete the w expression for the autoionization of water at 25 °C.
Answers
Answer:
Please mark brainlist
Explanation:
The equilibrium expression for this reaction is Kw = [H₃O⁺][OH⁻], where Kw is the autoionization constant for water. At 25°C, the value of Kw is 1.0 x 10⁻¹⁴.
earth has a low air pressure zone at the equator because what
Answers
Hey guys i needed some help on this

Answers
Based on the bond energies given, the standard enthalpy of the reaction, ΔH°(rxn) is -296 kJ.
What is standard enthalpy change of a reaction?The standard enthalpy change of a reaction is the energy changes involved when substances react under standard conditions to form products.
ΔH°(rxn) = sum of the bond energies of bonds being broken - sum of the bond energies of the bonds being formed.The standard enthalpy of the reaction, ΔH°(rxn) is calculated as follows:
Sum of energy of bonds broken = 839 + (2 × 413) + (432 × 2) = 2529 kJ
Sum of energy of bonds formed = 347 + (6 × 413) = 2825 kJ
ΔH°(rxn) = (2529 - 2825) kJ
ΔH°(rxn) = -296 kJ
Therefore, the standard enthalpy of the reaction, ΔH°(rxn) is -296 kJ.
Learn more about standard enthalpy at:https://brainly.com/question/25758173
What type of reaction is C3H6O + O2 --> CO2 + 3 H2O
Answers
Answer:
Combustion of propanone I believe
Explanation:
mass of 1×10^25 molecules of water
Answers
Answer:
1.E25 it is the answer the answer to mass of 1×10^25 molecules of water
Explanation:
this is just EXPLINATION find your answer using this
first divide the number of molecules by Avogadro's number 6.022*10^25
you will
l get no. of Moles of water
multiply the no. of Moles with mass of 1 Mole of water 18g per mole
if get answer you comment
you should try on your own you will understand better
Perform the following
mathematical operation, and
report the answer to the
correct number of significant
figures.
16.52 x 5.8 =
Answers
Answer:
95.8
Explanation:
The number comes out to be 95.816, but you round multiplcation to the lowest significant figure present in the problem. 16.52 has 4 sig figs, and 5.8 has 2 sig figs. Thus, your answer ends up being two significant figures.
Answer: 96
Explanation:
Which chemical species is responsible for the peaks near each wavelengths, 480 \pu{nm}nm and 430 \pu{nm}nm, respectively
Answers
The species that are responsible for the peaks near the wavelength are allura red and tartrazine.
What is a wavelength?It should be noted that wavelength simply means the distance between the identical points in the adjacent cycles of a waveform.
In this case, the chemical species is responsible for the peaks near each wavelengths include allura red and tartrazine.
It should be noted that allura red is used as a food dye and supplied as red sodium salt. Also, tartrazine is used as a food coloring agent.
Learn more about wavelength on:
https://brainly.com/question/10728818
kAnswer:
Explanation:
Which one of the following salts has the highest molar solubility in water?
Answers
Answer:
Correct option is
C
Ag
2
CO
3
; K
sp
=6.2×10
−12
(A) For CaCO
3
,S=
Ksp
=
8.7×10
−9
=9.3×10
−5
M
(B) For CuS,S=
Ksp
=
8.5×10
−45
=9.2×10
−23
M
(C) For Ag
2
CO
3
,S=(Ksp/4)
1/3
=(6.2×10
−12
/4)
1/3
=1.15×10
−4
M
(D) For Pb(IO
3
)
2
,S=(Ksp/4)
1/3
=(2.6×10
−13
/4)
1/3
=4×10
−5
M
Hence, Ag
2
CO
3
has the greatest molar solubility in pure water.
What’s is your average speed if you walk 50 meters in 100 seconds
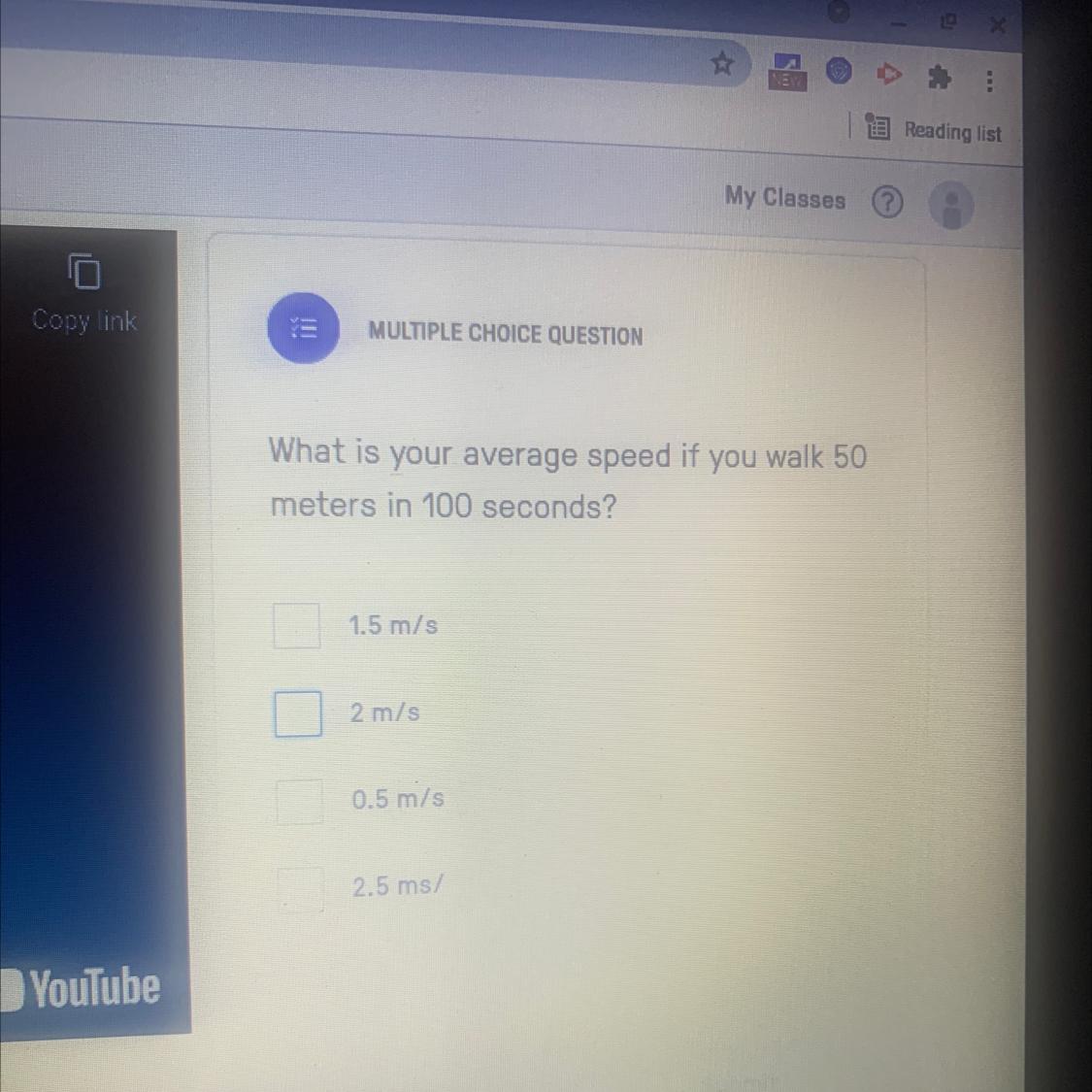
Answers
I'm pretty sure it's 1.5 mph, but i'm not 100% sure
Answer:
0.5 m/s
Explanation:
x = 50 m
t = 100 s
v = x / t
v = 50 / 100
v = 0.5 m/s
A hydrogen balloon has a
volume of 555 ml at 294 K. If
the balloon is cooled and the
volume decreases to 475 ml,
what is the new temperature?
Assume constant pressure.
Answers
Answer:
⇒ (400·3) / 294 = (P2·1) / 277
P2 = 1130.6 kPa
Explanation:
Where do the carbons and hydrogens go on this structure below?

Answers
The representation of carbons and hydrogens on the structure is shown on the attached image.
What is the given structure?The structure given is 3-Methylhexane. 3-Methylhexane is a branched-chain alkane with the molecular formula \(C_{7} H_{16}\). It is also known as 2,3-dimethylbutane or isoheptane.
The molecule consists of a six-carbon chain with a methyl group (-CH3) attached to the third carbon atom from one end, and another methyl group attached to the second carbon atom from the same end, giving it a branched structure.
3-Methylhexane is a colorless liquid with a boiling point of 90-91°C and a density of 0.68 g/cm3. It is commonly used as a reference compound in gas chromatography and as a blending component in gasoline to improve octane rating.
Learn about 3-Methylhexane here https://brainly.com/question/24165638
#SPJ1

HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
What is the name of Bel on the periodic table
Answers
Answer:
Nobelium or Beryllium