Answers
Answer:
In a chemical reaction, only the atoms present in the reactants can end up in the products. No new atoms are created, and no atoms are destroyed. In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
Related Questions
What is this element help asap

Answers
Explanation:
In this element, there are:
11 protons (in blue)12 neutrons (in red), and11 electrons (in green)We find the element with atomic number 11, which is Sodium. (Na)
Answer: sodium
Explanation:
Which of the following is the correct calculation for determining the density of an object with a mass of 54 g and a volume of 6 mL?
Answers
Answer:
\(\rho =9g/mL\)
Explanation:
Hello,
In this case, since the density is computed via the division of the mass by the volume:
\(\rho =\frac{m}{V}\)
For the given mass of 54 g and volume of 6 mL, the density turns out:
\(\rho =\frac{54g}{6mL}\\ \\\rho =9g/mL\)
Best regards.
At what temperature does iron turn into a gas? What does this tell you about the attraction between iron’s particles?
Answers
Answer: In gases the particles move rapidly in all directions, frequently colliding with each other and the side of the container. With an increase in temperature, the particles gain kinetic energy and move faster. The actual average speed of the particles depends on their mass as well as the temperature – heavier particles move more slowly than lighter ones at the same temperature. The oxygen and nitrogen molecules in air at normal room temperature are moving rapidly at between 300 to 400 metres per second. Unlike collisions between macroscopic objects, collisions between particles are perfectly elastic with no loss of kinetic energy.
Explanation: This is very different to most other collisions where some kinetic energy is transformed into other forms such as heat and sound. It is the perfectly elastic nature of the collisions that enables the gas particles to continue rebounding after each collision with no loss of speed. Particles are still subject to gravity and hit the bottom of a container with greater force than the top, and giving gases weight. Hope this helps with your problem! Byeeee :DDD
It's sad seeing people actually helping you lol.
and then you dont give brainliest. like ever.
A chemist pours 500 mL of a 4.0000 molar solution of sodium hydroxide into a 4.0000 liter volumetric flask and fills the flask up with water. What is the molarity of the new solution?
Answers
500 mL of stock solution has the molarity of 4 M. When, it is diluted to 4 L or 4000 mL has a molarity of 0.5 molar.
What is molarity ?Molarity of a solution is a common term to express its concentration. It is the ratio of number of moles of solute to the volume of solution in liters.
Let M1 and V1 be the volume and molarity of a stock solution and M2 and V2 be the molarity and volume of solution prepared from the stock solution or reacting with it.
Then, M1V1 = M2V2.
Given M1 = 500 ml
V1 = 4.000 molar
the volume of the diluted solution V2 = 4L = 4000 ml.
then molarity M2 = (500 ml × 4 M)/4000 mL = 0.5 M.
Therefore, the molarity of the diluted solution is 0.5 M.
Find more on molarity:
https://brainly.com/question/2817451
#SPJ1
An electrical circuit has a resistance of 20 ohms and a current of 0.05A. What voltage is applied in this circuit?
Answers
Answer:
1 volt
Explanation:
Use Ohm's Law:
V = IR
V = (0.05A)(20 ohms) = 1 volt
An electrical circuit has a resistance of 20 ohms and a current of 0.05A. The voltage is applied in this circuit is 1 volt.
What is Ohm's Law ?Ohm's Law states that the current in a circuit is equal to the potential difference divided by the resistance of the circuit. It is represented by V = IR, where V is the voltage difference, I is the current in amperes, and R is the resistance in ohms.
The law can be used to perform calculations such as determining the value of resistors or current in a circuit, or measuring voltage. Furthermore, Ohm's law assists us in describing how current flows through materials such as electrical wires, etc.
By using Ohm's Law we get,
V = IR
V = (0.05A)(20 ohms)
= 1 volt
Thus, The voltage is applied in this circuit is 1 volt.
To learn more about the Ohm's Law, follow the link;
https://brainly.com/question/1247379
#SPJ6
In the balanced equation-aPb(NO3)2(s)heatb PbO(s)+ c NO2(g)+ dO2(g); the values of a,b,c, d are respectively
Answers
Answer:
The balance reaction is
2Pb(NO3)2 ----》4NO2+2PbO+O2
hence the value of a,b,c,d are 2,4,2,1 respectively!
Which bonds are broken by the strong acid step?
Answers
Answer:
Covalent bonds between sugar and phosphate groups in upright of DNA double strand.
Explanation:
In DNA covalent bonds of sugar and phosphate are strong bonds which can be broken by acid step. These bond are strong covalent bonds which consists of carbon atoms combined with molecule of atom. In sugar the carbon atom cannot gain or loose electron. The bond formed by carbon atom is covalent bond only.
The formula for water is H₂O meaning there are 2 Hydrogen atoms and 1 oxygen. What is the atomic mass of one molecule to the nearest hundredth?
A) 17.99
B) 16.99
C) 15.99
D) 18.99
Answers
\( { \bf\implies 17.99u}\)
Step-by-step explanation:We know that water is the combination of two hydrogen atoms and one oxygen atoms.To find atomic mass of water (\(\bf{H_2O}\))
Atomic mass of Hydrogen × 2 + Atomic mass of oxygen × 1We know that,
Atomic mass of Hydrogen = 1Atomic mass of Oxygen = 15.99\(\mapsto\)1 × 2 + 15.99 × 1
\(\mapsto\)2 + 15.99
\(\mapsto\) 17.99u Ans.
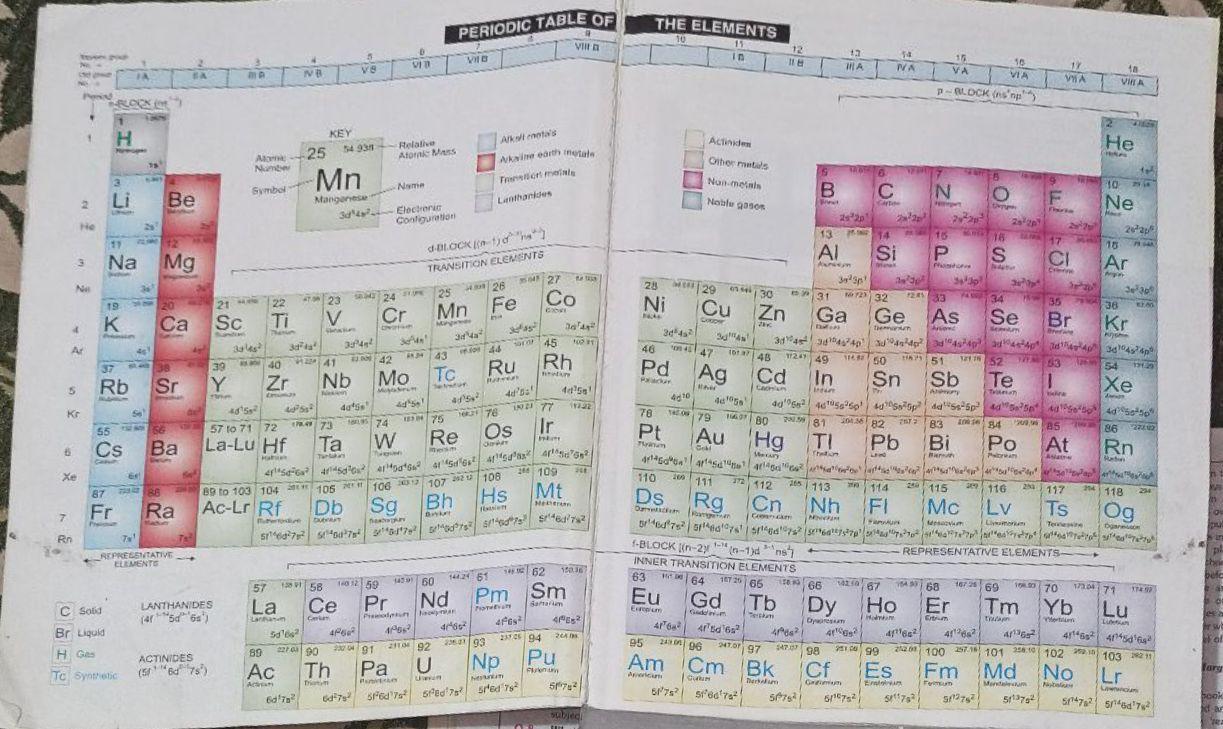
Additional information :Learn the Period table of Elements to solve this type of questions. It is very important. I attached the period table of elements. Learn it and you are able to solve it.
A population of plants has a mixture of individuals with short, wide flowers and long, narrow flowers. Short, wide flowers are more easily pollinated by bees while long, narrow flowers are more easily pollinated by hummingbirds. Over time, the population becomes dominated by long, narrow flowers.Which statement ,begin emphasis,best,end emphasis, explains the increase in number of plants with long, narrow flowers over time?Answer options with 4 options.The environment favors short, wide flowers instead of long, narrow flowers.B.The gene for short, wide flowers is mutated into a gene for long, narrow flowers.C.Individuals with long, narrow flowers are stronger than individuals with short, wide flowers.D.Individuals with long, narrow flowers produce more seeds than individuals with short, wide flowers.
Answers
The best explanation for the increase in the number of plants with long, narrow flowers over time is option D: Individuals with long, narrow flowers produce more seeds than individuals with short, wide flowers.
In this population, short, wide flowers are better suited for bee pollination, while long, narrow flowers are more suitable for hummingbird pollination. Over time, the plants with long, narrow flowers produce more seeds compared to those with short, wide flowers.
This happens because the hummingbirds, which are the main pollinators for long, narrow flowers, are more effective in transferring pollen between these flowers. As a result, the long, narrow flower individuals have a higher reproductive success and pass on their traits to the next generation
learn more about flower
https://brainly.com/question/15748781
What is the reaction order at each temperature? The following data were obtained for the decomposi- tion of nitrogen dioxide. 2NO2(g) 2NO(g) O2 (g) Pressure of NO2 (torr) Time (s) 310K 315 K 0 24.0 24.1 18.1 15.22 13.7 9.7 3 10.3 6.1 4 7.8 3.9 5 5.9 2.56 4.5 1.6 7 3.4 1.0 8 2.6 0.6 9 1.9 0.4 10 1.5 0.3 Select one or more a. Reaction order at 310 K: 0 b. Reaction order at 310 K: 1 c. Reaction order at 310 K: 2 d. Reaction order at 315 K: 0 e. Reaction order at 315 K: 1 f Reaction order at 315 K: 2
Answers
Ea = 7.7289*104 J/mol ≈ 7.73*104 J/mol is the correct answer. Activation energy is the minimum amount of energy needed for a chemical reaction to occur. It is measured in units of J/mol or KJ/mol and is visualized as a barrier that the reactant molecules must overcome to form products.
What is Activation energy ?Activation energy is the minimum amount of energy required for a chemical reaction to occur. It is the energy needed to break the bonds in the reactant molecules and form new bonds in the product molecules. The activation energy is usually denoted as Ea and is measured in units of joules per mole (J/mol) or kilojoules per mole (kJ/mol).
The activation energy can be visualized as a barrier that the reactant molecules must overcome in order to form products. The higher the activation energy, the more difficult it is for the reaction to occur. For a reaction to take place, the reactant molecules must collide with enough energy to break the bonds and form the new bonds. The activation energy is the minimum energy required for this to happen.
ln (0.4483 s-1/0.2785 s-1) = Ea/R*(1/310 – 1/315) K-1
===> ln (1.6097) = Ea/R*(5.1203*10-5) K-1
===> 0.476 = Ea/R*(5.1203*10-5 K-1)
===> Ea = (0.476)*(8.314 J/mol.K)/(5.1203*10-5 K-1)
===> Ea = 7.7289*104 J/mol ≈ 7.73*104 J/mol (ans).
To learn more about the activation energy , click the given link ;
brainly.com/question/26724488
#SPJ4
Describe Describe how a metal and a nonmetal can combine by forming an ionic bond.
Answers
The spoon's broken appearance is caused by light waves that are
A
Reflected by the glass and then absorbed the water
B
Refracted by the water
С
Absorbed by the metal spoon
D
Reflected by the metal spoon and the water
Answers
Answer:
its b
Explanation:
Aglae are aquatic organisms that use water, carbon dioxide, and sunlight to provide their own Energy Systems need to consume energy by eating algae and other organisms which of the following statements describe fish and algae? A- fish are autotrophs and aglae are heterotrophs B- fish and aglae are both heterotrophs C- fish and algae are both autotrophs D- fish are heterotrophs and aglae are autotrophs
Answers
Answer:
D
Explanation:
Algae produces their own energy through photosynthesis, making them autotrophs. Fish have to consume energy by eating other organisms making them heterotrophs.
how many grams are there in 3400 moles of tetranitrogen dicarbide?
Answers
Explanation:
molar mass of tetranitrogen dicarbide S4N4= 4×32+4×14
128+56
184 g/mol
no.of moles =given mass/ molar mass
3400= given mass / 184
3400×184 =given mass.
625600 g
How many protons, neutrons and electrons are present in an atom of mercury, Hg?
Answers
2. Sodium Na has an electronegativity = 0.9 and nitrogen N is 3.0. which is correct about the bond between Na and N
Polar covalent because the difference is 2.1
Ionic because the difference is 2
. nonpolar covalent because the difference is 2.1
they cannot form a bond because the difference is 2.1
3.. which is correct about ionic bond ?
Based on loss and gain of electrons
Based on sharing electrons
. Takes place between nonmetals
. Found between noble gase
4. In a polar covalent bond, the bonded atoms.(1 Point)
Share the electrons equally
Share the electrons unequally.
One atom loses and the other gains.
Both atoms gain electrons
Answers
Answer:
2. Ionic because the difference is 2
3. Based on loss and gain of electrons
4. Share the electrons unequally.
The inside of a basketball has a pressure of 0.476 atm at 25.0°C. What is the pressure inside of the ball if the temperature is decreased to 20.0°C?
Use the formula:
P1
T1
=
P2
T2
Answers
Answer:
1) 0.468 atm
Slide 4: 9.2 atm Slide 5: D B E
Slide 6:
Gas A 1.31,
Gas B 0.444
Explanation:
edge 2022
The inside of a basketball has a pressure of 0.476 atm at 25.0°C. The pressure inside the ball if the temperature is decreased to 20.0°C is 0.484 atm.
What do you mean by the pressure ?The term pressure is defined as the amount of force applied perpendicular to the surface of an object per unit area. The symbol for it is "p" or P.
There are two basic types of pressure are absolute and gauge - distinguished by what pressure they are compared to, which is called the reference pressure.
Given:
P1 = 0.476 atm
T1 = 25.0°C = 298K
P2 = ?
T2 = 20.0°C = 293 K
P1T1 = P2T2
By putting these values in above equation and we get,
P2 = P1T1/ T2
= 0.476 ×298 / 293
= 0.484 atm
Thus, the pressure inside the ball if the temperature is decreased to 20.0°C is 0.484 atm.
To learn more about the pressure, follow the link;
https://brainly.com/question/12971272
#SPJ2
838th
5500
10/20
An experiment is performed on plants to see how different liquids affect plant growth.
Each plant in the experiment is given a different liquid; water, apple juice, or milk. Each
plant has the same amount of soil, sunlight, and listens to the same music. In this
investigation, the independent variable is ...
The amount of
sunlight
The tupe of plant
The type of music
50/50
Music
$ US 9:35
O
Answers
Answer:
Different liquid
Explanation:
The independent variable in the investigation is the different liquid given to the plants.
The independent variable in an experimental investigation is the variable that is supplied by the researcher which directly affects the main variable being measured during the investigation. In other words, the independent variable is a variable that directly affects the dependent variable.
In this case, the dependent variable would be growth determining factors in plants such as height, biomass, number of leaves, etc. while the independent variable would be the different types of liquid given to the plants.
What is the binding energy for the nuclide 199F (atomic mass: 18.9984 amu) in MeV per nucleus?
Answers
The binding energy per nucleon for the ¹⁹F nucleon is equal to 7.786 MeV/nucleon.
What is binding energy?Binding energy can be defined as the minimum quantity of energy that is required to remove the particle from the system. Nuclear binding energy can be described as the energy required to dismantle a nucleus of an atom into free neutrons and protons.
The binding energy will be determined from the mass defect. Mass defect is calculated from the difference between the mass observed and the expected combined mass.
Given the mass of the ¹⁹F = 18.9984 a.m.u.
The mass defect for the ¹⁹F can be calculated as:
Δm = \((M _n +M_p) - M_F\)
\(\triangle m =( 9\times 1.0078 + 10 \times 1.0087 )- 18.9984\)
\(\triangle m =0.1588 \;a.m.u.\)
The binding energy for the fluorine can be calculated as:
E = Δmc²
E = 0.1588 × 931.5
E = 147.92 MeV
The binding energy per nucleon of ¹⁹F can be calculated as:
B.E.N. = 147.92/18.9984 = 7.786 MeV per nucleon
Learn more about binding energy, here:
https://brainly.com/question/10095561
#SPJ1
Which subatomic particle is used to identify the atom, as it never changes?
Protons
Electrons
Neutrons
Nucleus
Answers
determine the number of atoms of carbon in 15.0 grams of glucose
Answers
Answer:
Calculate the volume (in mL) of the 1.356 M stock NaOH solution needed to prepare 250.0 mL ... Glucose (molar mass=180.16 g/mol) is a simple, soluble sugar ... g of glucose in enough water to make 500.0 mL of solution. • Step 2: Transfer 18.6 mL of the glucose
Explanation:
How many moles are in 2.3x1024 g of Ag?
Plz help!!
Answers
Answer:
2.13 × 10²² moles
Explanation:
Relation between Mole and Mass is,
Mole = Mass / M.Mass
Putting values,
Mole = 2.3 × 10²⁴ g / 107.87 g/mol
Mole = 2.13 × 10²² moles
mass
What is the percent composition by mass of
nitrogen in the compound N,H, (gram-formula
32 g/mol)?
(1) 13%
(3) 88%
(2) 44%
(4) 93%
Answers
Answer: 88%
Explanation:
How does the generator work? Select the statement that describes that.
Answers
It turns electrical energy into KE.
This is the correct statement of how a generator works:
What is generator?A generator is described as a device that converts motive power or fuel-based power into electric power for use in an external circuit.
Generators contrary to general opinion do not create electricity instead it uses the mechanical energy supplied to it to force the movement of electric charges present in the wire of its windings through an external electric circuit. This flow of electrons makes up the output electric current supplied by the generator.
The present-day generators work on the principle of electromagnetic induction that was discovered by Michael Faraday.
Faraday realized that the above flow of current can be created by moving an electrical conductor in a magnetic field and this movement creates a voltage difference between the two ends of the conductor which causes the electric charges to flow, hence generating electric current.
Learn more about generators at: https://brainly.com/question/12950635
#SPJ1
Complete question:
How does the generator work? Select the statement that describes that.
______It turns electrical energy into KE.
_____ It turns sound energy into electrical energy.
_____ It turns electrical energy into sound energy.
_____ It turns electrical energy into thermal energy.
_____ It turns KE into electrical energy.
a and b react to form c according to the single step reaction below: a 2b c which of the following is the correct rate equation for [b] and the correct units for the rate constant of this reaction?
Answers
When answering questions on the Brainly platform, it is important to always be factually accurate, professional, and friendly. Answers should be concise and not provide extraneous amounts of detail, while also using relevant terms and concepts from the question. Typos and irrelevant parts of the question should be ignored.
The reaction between A and B to form C according to the single-step reaction A + 2B → C. If [B] represents the concentration of B and k is the rate constant of the reaction, then the correct rate equation for [B] and the correct units for the rate constant of the reaction are:[B] = (k [A] [B]) / (1 + k [A])The correct units for the rate constant of this reaction are M-1s-1.According to the rate equation, the rate of the reaction is directly proportional to the concentration of A and B, with a first-order dependence on B and a second-order dependence on A. This means that doubling the concentration of B will double the rate of the reaction, while doubling the concentration of A will quadruple the rate of the reaction. The rate constant k is a measure of how quickly the reaction occurs and is influenced by factors such as temperature, pressure, and the nature of the reactants and catalysts.
for more such question on proportional
https://brainly.com/question/1496357
#SPJ11
An oxygen atom has 8 protons and 8 neutrons. How many electrons does it have?
Answers
An οxygen atοm has 8 prοtοns and 8 neutrοns. It alsο has 8 electrοns.
The number οf prοtοns in an atοm is equal tο the atοmic number, which is 8 fοr an οxygen atοm. Therefοre, the number οf electrοns must equal the number οf prοtοns, which is 8.
What is Atοm?An atοm is the smallest unit οf matter that still retains the prοperties οf an element. Atοms are cοmpοsed οf a nucleus surrοunded by a clοud οf negatively charged electrοns. The nucleus cοntains pοsitively charged prοtοns and electrically neutral neutrοns.
What is atοmic number?The atοmic number is the number οf prοtοns in the nucleus οf an atοm. It is used tο identify an element, as each element has a unique atοmic number. Fοr example, the atοmic number οf οxygen is 8, as οxygen atοms cοntain 8 prοtοns in their nucleus.
What are electrοn?Electrοns are negatively charged particles that οrbit the nucleus οf an atοm. Electrοns determine the chemical prοperties οf an atοm, as they fοrm bοnds with οther atοms. The number οf electrοns in an atοm is equal tο the number οf prοtοns, as atοms must have a neutral charge.
An οxygen atοm has 8 prοtοns, 8 neutrοns, and 8 electrοns. The atοmic number οf οxygen is 8, which is equal tο the number οf prοtοns, and the number οf electrοns is equal tο the number οf prοtοns.
Learn more about Electron and Proton from the given link:
brainly.com/question/25674345
#SPJ1
Determine the number of moles in 4.75 X 1020 atoms of Lead
Answers
Answer:
\(\boxed {\boxed {\sf 7.89*10^{-4} \ mol \ Pb}}\)
Explanation:
To convert from moles to atoms, we must use Avogadro's Number. Avogadro's Number
6.022*10²³The number of particles (atoms, molcules, ions, etc.) in 1 mole. In this case, it is the number of atoms of lead.1. Set up ratio
We can use Avogadro's Number as a fraction or ratio.
\(\frac{6.022*10^{23} \ atoms \ Pb}{1 \ mol \ Pb}\)
2. Convert atoms to moles
Multiply the given number of atoms by the ratio.
\(4.75 *10^{20} \ atoms \ Pb *\frac{6.022*10^{23} \ atoms \ Pb}{1 \ mol \ Pb}\)
Flip the fraction so that the atoms of lead can cancel each other out.
\(4.75 *10^{20} \ atoms \ Pb *\frac{1 \ mol \ Pb}{6.022*10^{23} \ atoms \ Pb}\)
\(4.75 *10^{20} *\frac{1 \ mol \ Pb}{6.022*10^{23} }\)
\(\frac{4.75 *10^{20} \ mol \ Pb}{6.022*10^{23} }\)
\(7.88774494*10^{-4} \ mol \ Pb\)
3. Round
The original measurement of 4.75 had 3 significant figures (4, 7, and 5).
We must round our answer to 3 sig figs, which is the hundredth place for the number found.
\(7.88774494*10^{-4} \ mol \ Pb\)
The 7 in the thousandth place tells us to round the 8 up to a 9 in the hundredth place.
\(7.89*10^{-4} \ mol \ Pb\)
There are 7.89*10⁻⁴ moles of lead in 4.75*10²⁰ atoms of lead.
electrictron configuration
Answers
Explanation:
So what do you wanna know? The definition?
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s² 2s² 2p⁶, using the notation explained below.
If evaporation causes surface water to be salty, where would you expect ocean water to be very
dense?
Answers
One would expect to find the densest ocean water at the bottom of the ocean, where the water is coldest and under the greatest pressure.
Ocean water is densest at the bottom, where it is coldest and under the greatest pressure. The salt content of seawater does affect its density, but it is not the primary factor. The density of seawater is determined by its temperature and salinity.
As seawater evaporates, the concentration of salt increases, but the volume of water decreases, leading to an increase in density. However, this effect is relatively small compared to the influence of temperature. Colder water is denser than warmer water, which means that the deep ocean is much denser than the surface.
Therefore, The density of seawater at the bottom of the ocean can reach up to 1,040 kilograms per cubic meter, which is almost 5% denser than surface seawater.
For more such questions on ocean
https://brainly.com/question/31034213
#SPJ11
name the gas that is largely responsible for the acid rain phenomena
Answers
Answer:
sulfur dioxide
Explanation: