Answers
Answer:
What would be the formal charge of N in NO2?
Explanation:
The nitrogen gets two electrons for the double bond, one from the single bond and one from the half-filled orbital associated with it. That's a total of 4 and gives N a +1 formal charge since itneeds 5 to be neutral. The Rules for Writing Lewis Structures has some discussion of formal charge in it.
Related Questions
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.
consider the reaction between mg s and hcl(aq) to produce aqueous magnesium chloride and hydrogen gas. How many liters of hydrogen gas at STP will be produced when 12.15 g of magnesium reacts with an excess of hydrochloric acid
Answers
Answer:
11.2L is the volume of hydrogen gas produced.
Explanation:
The reaction of Mg with HCl is:
Mg + 2HCl → MgCl₂ + H₂
Based on the reaction the moles of Magnesium are equal to moles of hydrogen produced, that is:
Moles Mg -Molar mass: 24.305g/mol-:
12.15g Mg * (1mol / 24.305g) = 0.500 moles of Mg = Moles of H₂
Now, using PV = nRT, where:
P is pressure -1atm at STP-
V is volume -Our incognite-
n are moles -0.500 moles of H₂-
R is gas constant -0.082atmL/molK-
And T is temperature -273.15K-:
1atm*V = 0.500mol*0.082atmL/molK*273.15K
V = 11.2L is the volume of hydrogen gas produced.
Answer: 11.2
Explanation:
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
When 6.0 mol Al react with 13 mol HCl what is the limiting reactant and how many moles can be formed
2Al + 6HCl - 2AlCl3 3H2
Answers
The limiting reactant in the reaction is HCl
The amount of products that can be formed in the reaction is:
4.33 moles of AlCl₃
6.5 moles of H₂
What is a limiting reactant?A limiting reactant is a reactant that is used up in a reaction.
The limiting reactant when once used up, and the reaction stops. Thus, the limiting reactant produces the least amount of product from the reactants that are present.
Considering the given reaction:
2 Al + 6 HCl ---> 2 AlCl₃ + 3H₂
The mole ratio of the reactants aluminum and hydrochloric acid is 2 : 6
Hence, 13 moles of HCl will require 13 * 2/6 moles of Al = 4.33 moles of Al
Hence, HCl is the limiting reactant.
The amount of products that can be formed is:
AlCl₃ = 13 * 2/6
AlCl₃ = 4.33 moles
H₂ = 13 * 3/6
H₂= 6.5 moles
Learn more about limiting reactants at: https://brainly.com/question/19033878
#SPJ1
Which metal does not form cations of differing charges?
Answers
Transition metals
Most transition metals differ from the metals of Groups 1, 2, and 13 in that they are capable of forming more than one cation with different ionic charges. As an example, iron commonly forms two different ions
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
what is the energy of a photon that emits a light frequency of 6.42X10(14)HZ? got it... 4.25 X10 (-19)J
Answers
Among electromagnetic waves, UV rays are most dangerous because exposure to these radiation cause serious problems in living organism. Therefore, 4.28×10⁻¹⁹J is the energy of a photon that emits a light frequency of 6.67×10⁻³⁴ Hz.
What is electromagnetic wave?Electromagnetic wave is a wave which contain two component one is electric component and other is magnetic component. The electric and magnetic component are perpendicular to each other. There are so many wave that comes under electromagnetic wave like infrared wave , radio wave.
There is a relation between energy of wave. frequency of wave, and wavelength of wave
Mathematically,
E=h×ν
where,
E = energy of electromagnetic wave =?
h is planks constant having value 6.67×10⁻³⁴js
ν= frequency of photon=6.42X10¹⁴ Hz
Substituting all the given values in the above equation, we get
E= 6.67×10⁻³⁴×6.42X10¹⁴
E=4.28×10⁻¹⁹J
Therefore, 4.28×10⁻¹⁹J is the energy of a photon that emits a light frequency of 6.67×10⁻³⁴ Hz.
To know more about electromagnetic wave, here:
https://brainly.com/question/12289372
#SPJ1
Haaaaaaaalllllllpppppp
Name 2 Triangular Items that are something you can hold. And then name those items' Length, Width, Height, Area, or Volume.
Answers
Answer:
ice creem cone candy
Explanation
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
If 4.80 mol Ca mixed with 2 mol N2, which is the limiting reactant? 3Ca (s) + N2 (g) Ca3N2 (s)
Answers
Two samples of carbon come into contact. A heat transfer will occur between sample A and sample B. What must be
true for heat to transfer from sample A to sample B?
O The average kinetic energy of A is greater than that of B.
O The average kinetic energy of B is greater than that of A.
O The average kinetic energy of both samples is equal.
O The average kinetic energy does not determine the direction of heat transfer.
Answers
The direction of heat transfer between two samples of carbon depends on their temperature difference, and not solely on their average kinetic energy. While the average kinetic energy of a substance is related to its temperature, it is not the determining factor for the direction of heat transfer.
When two samples of carbon come into contact, a heat transfer will occur between sample A and sample B. The direction of heat transfer is dependent on the temperature difference between the samples. Heat transfer always flows from a hotter object to a cooler object, so if sample A is hotter than sample B, heat will flow from A to B. If sample B is hotter than sample A, heat will flow from B to A.
The average kinetic energy of the molecules in a substance is related to its temperature. The higher the average kinetic energy, the higher the temperature of the substance. However, the average kinetic energy does not determine the direction of heat transfer.
It is possible for a substance with a lower average kinetic energy (and therefore a lower temperature) to transfer heat to a substance with a higher average kinetic energy (and therefore a higher temperature). This can occur if the substance with the lower temperature has a greater heat capacity, which means it can absorb more heat without a significant increase in temperature.
for more questions on kinetic energy
https://brainly.com/question/25959744
#SPJ8
In a chemical reaction
Zn(NO3)2 + NO2 + H₂O
a) What is redox reaction?.
b) Balance the reaction by oxidation number or ion electron method.
Answers
A redox reaction is a reaction in oxidation or the loss of electrons occurs simultaneously with reduction involving a gain of electrons.
The balanced equation of the redox reaction by the oxidation number method is as follows: Zn + 2HNO₃ ----> Zn(NO₃)₂ + 2NO₂ + H₂O
What is the balanced equation of the redox reaction?The complete equation of the redox reaction is given below as follows:
Zn + HNO₃ ----> Zn(NO₃)₂ + NO₂ + H₂OTo balance the chemical reaction by oxidation number, we need to ensure that the total change in oxidation numbers for each element is zero on both sides of the equation.
Let's assign the oxidation numbers to the elements:
In Zn(NO₃)₂, the oxidation number of Zn is +2, and the oxidation number of each NO₃ group is -1.
In HNO₃, the oxidation number of H is +1, the oxidation number of N is +5, and the oxidation number of each O in NO₃ is -2.
On the product side, the oxidation number of Zn is +2, and the oxidation number of each NO₃ group is -1. The oxidation number of N in NO₂ is +4, and the oxidation number of each O is -2. The oxidation number of H in H₂O is +1, and the oxidation number of O is -2.
Now, let's balance the reaction by considering the changes in oxidation numbers:
Zn: 0 → +2
H: +1 → 0
N: +5 → +4
O: -2 → -2
To balance the oxidation numbers, we need two NO₂ molecules on the product side. The balanced equation is:
Zn + 2HNO₃ ----> Zn(NO₃)₂ + 2NO₂ + H₂O
Learn more about redox reactions at: https://brainly.com/question/21851295
#SPJ1
Number 3
The question is: What are the formulas for Ions and Compunds formed?
Ca, CL
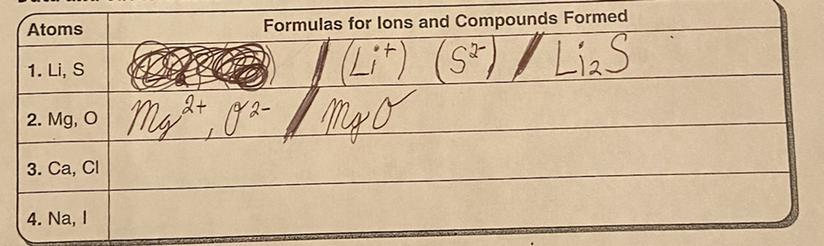
Answers
Answer:
hope this help u! sorry late answer

what is the percent composition of muscovite mica. Its chemical formula is
(KF)2(Al2O3)3(SiO2)6(H2O)
Answers
Answer:
\(\boxed{\text{0.25 \% H, 43.96 \% O, 4.75 \% F, 20.22 \% Al, 21.05 \% Si, 9.77 \% K}}\)
Explanation:
The oxide formula is (KF)₂(Al₂O₃)₂(SiO₂)(H₂O).
Rewrite it as a molecular formula — H₂O₂₂F₂Al₆Si₆K₂
The formula for the mass percent of an element is
\(\text{Mass \%} = \dfrac{\text{mass of element}}{\text{mass of compound}} \times 100 \, \%\)
We can set up a table to calculate the percent of each element.
\(\begin{array}{rrrrr}\textbf{Atom} & \textbf{No.} &\textbf{MM/u} & \textbf{Mass/u} & \mathbf{\%} \\\text{H} & 2& 1.01 & 2.02& 0.25 \\\text{O} &22 & 16.00 & 351.98 & 43.96 \\\text{F} & 2 & 19.00 & 38.00 & 4.75 \\\text{Al} & 6 & 26.98 & 161.89 & 20.22 \\\text{Si} & 6 & 28.08 & 168.51 & 21.05 \\\text{K} & 2 & 39.10 & 78.20 & 9.77 \\& & \text{TOTAL =} & \mathbf{800.60} & \mathbf{100.00} \\\end{array}\\\)
\(\text{The percent composition of muscovite mica is}\\ \boxed{\textbf{0.25 \% H, 43.96 \% O, 4.75 \% F, 20.22 \% Al, 21.05 \% Si, 9.77 \% K}}\)
Given the equation, Na+ + CI→ NaCl, calculate the mass of NaCl produced by the reaction of 54.2 g of chloride ions with an excess of sodium. A. 165.0 g B. 17.8 g c. 72.0 g D. 219.2 g E. 89.3 g
Answers
The mass of NaCl produced by the reaction of 54.2 g of chloride ions with an excess of sodium is 89.51 grams.
How to calculate mass using stoichiometry?Stoichiometry is the concentration of a substance in solution, expressed as the number of moles of solute per litre of solution.
According to this question, 1 mole of sodium reacts with 1 mole chloride ion to produce 1 mole of sodium chloride.
54.2 grams of chlorine is equivalent to 1.53 moles. Hence, 1.53 moles of sodium chloride will be produced.
1.53 moles of sodium chloride is equivalent to 89.51 grams.
Learn more about stoichiometry at: https://brainly.com/question/9743981
#SPJ1
Which part of a calcium atom in the ground state is represented by the dots in its Lewis electron-dot diagram?
Answers
Answer:
There are two dots are present on the left side of calcium atom which represents the unpaired electrons present in the outermost shell. This structure is presented by Lewis in order to show the number of unpaired electrons in the atom.
Explanation:
Calculate each of the following quantities:
a) Molarity of a solution prepared by diluting 27.0 cm3
of 0.150 M potassium chloride to
150.0 cm3
b) Molarity of a solution prepared by diluting 35.71 cm3
of 0. 0756 M ammonium
sulfate to 500 cm3
c) Final volume of a 0.05M solution prepared by diluting 10.0 cm3
of 0.155 M lithium
carbonate with water
Answers
Answer:
A. 0.027 M
B. 0.0054 M
C. 31 cm³
Explanation:
A. Determination of the final concentration (Molarity)
Initial Volume (V₁) = 27 cm³
Initial concentration (C₁) = 0.150 M
Final volume (V₂) = 150 cm³
Final congratulation (C₂) =?
C₁V₁ = C₂V₂
0.150 × 27 = C₂ × 150
4.05 = C₂ × 150
Divide both side by 150
C₂ = 4.05 / 150
C₂ = 0.027 M
Thus, the final concentration of the solution is 0.027 M
B. Determination of the final concentration (Molarity)
Initial Volume (V₁) = 35.71 cm³
Initial concentration (C₁) = 0.0756 M
Final volume (V₂) = 500 cm³
Final congratulation (C₂) =?
C₁V₁ = C₂V₂
0.0756 × 35.71 = C₂ × 500
Divide both side by 500
C₂ = (0.0756 × 35.71) / 500
C₂ = 0.0054 M
Thus, the final concentration of the solution is 0.0054 M
C. Determination of the final volume.
Initial Volume (V₁) = 10 cm³
Initial concentration (C₁) = 0.155 M
Final congratulation (C₂) = 0.05 M
Final volume (V₂) =?
C₁V₁ = C₂V₂
0.155 × 10 = 0.05 × V₂
1.55 = 0.05 × V₂
Divide both side by 0.05
V₂ = 1.55 / 0.05
V₂ = 31 cm³
Thus, the final volume of the solution is 31 cm³
Determine the value of Kc for the following reaction, if the equilibrium concentrations are as follows: [N2]eq = 2.66 M, [H2]eq = 0.64 M, [NH3]eq = 3.34 M.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
Answers
The value of Kc for the given reaction is 0.0579 (rounded to four decimal places).
The formula for the equilibrium constant, Kc, of a reaction is given by the ratio of the product of the concentrations of the products raised to their respective stoichiometric coefficients to the product of the concentrations of the reactants raised to their respective stoichiometric coefficients.
The stoichiometric coefficients are the coefficients in the balanced chemical equation.
To determine the value of Kc for the reaction given by the following chemical equation:N2(g) + 3 H2(g) ⇌ 2 NH3(g)
we first need to write the expression for Kc.
The expression for Kc is given by the following formula:Kc = [NH3]² / [N2][H2]³.
We are given the equilibrium concentrations as follows:[N2]eq = 2.66 M[H2]eq = 0.64 M[NH3]eq = 3.34 M
We can substitute these values into the expression for Kc and obtain the following:Kc = (3.34)² / (2.66)(0.64)³ = 0.0579 (rounded to four decimal places).
For more such questions on Kc
https://brainly.com/question/15225808
#SPJ8
Joan wrote a science fiction story where the people only texted each other, and never talked. They still had vocal chords, but they could no
longer make sounds. Their vocal chords were
Answers
Answer:
Vestigial
Explanation:
The retention of genetically determined traits or structures that have partially or completely lost their ancestral purpose in a specific species is known as vestigiality. In most cases, evaluating the vestigality requires comparison with comparable traits in closely related species.
does the molecule which have C2 axis perpendicular to the Cn axis have mirror plane perpendicular to the Cn axis ?
Answers
No, a molecule with a \(C_2\)axis perpendicular to the \(C_n\)axis does not necessarily have a mirror plane perpendicular to the \(C_n\)axis.
The presence of a \(C_2\)axis perpendicular to the \(C_n\)axis implies that the molecule possesses rotational symmetry around the \(C_n\)axis. However, the presence of a mirror plane is determined by the presence of an additional symmetry element in the molecule.
A mirror plane is a symmetry element that divides the molecule into two halves, with one half being the mirror image of the other. In order for a mirror plane to be present perpendicular to the \(C_n\)axis, there needs to be an additional symmetry element that produces the reflection symmetry.
While a molecule with a \(C_2\) axis perpendicular to the \(C_n\)axis has rotational symmetry, it does not necessarily possess reflection symmetry. For example, consider a molecule with a \(C_2\)axis perpendicular to a \(C_3\)axis.
The rotational symmetry is evident, as the molecule can be rotated by 120 degrees around the \(C_3\) axis and still appear the same. However, this molecule does not possess a mirror plane perpendicular to the \(C_3\)axis.
The presence of a mirror plane perpendicular to the \(C_n\)axis depends on the specific molecular geometry and arrangement of atoms. It is possible for a molecule to possess both rotational symmetry and a mirror plane perpendicular to the \(C_n\)axis, but it is not a general rule.
For more such questions on perpendicular visit:
https://brainly.com/question/23828050]
#SPJ8
i need help with unit conversion
Answers
Answer:
what is it?
Explanation:
you haven't said?
If you had 0.08841 mol of sucrose present in a 625 mL aqueous solution, what would be the molarity of the solution? (Remember that molarity is defined in terms of liters of the solution!)
Answers
The molarity of the solution with 0.08841 moles of sucrose in 625 mL of aqueous solution is 0.1414 M.
Given the number of moles of sucrose (n) = 0.08841mol
The volume of aqueous sucrose solution (V) = 625mL = 0.625L
Let the molarity of solution = M
Molarity (M) is the number of moles of solute per liter of solution. To find the molarity of a solution, we need to divide the number of moles of solute by the number of liters of solution.
Therefore, the molarity of the solution with 0.08841 moles of sucrose in 625 mL of aqueous solution can be calculated as follows:
Molarity (M) = (moles of solute(n)) / (Volume of solution(V))
M = (0.08841 mol sucrose) / (0.625 L solution)
M = 0.1414M sucrose
To learn more about molarity click here https://brainly.com/question/8732513
#SPJ1
The tomato is dropped. What is the velocity, v
, of the tomato when it hits the ground? Assume 86.0 %
of the work done in Part A is transferred to kinetic energy, E
, by the time the tomato hits the ground.
Express your answer with the appropriate units.
Answers
To determine the tomato's velocity when it hits the ground, we need more information. Specifically, we need the height from which the tomato was dropped and the tomato mass.
Without these details, it is impossible to calculate velocity accurately. The velocity of an object when it hits the ground depends on factors such as the height of the fall, the mass of the object, and any forces acting on it during the fall (such as air resistance).
If you can provide the necessary information, I can help you calculate the velocity of the tomato when it hits the ground.
62 minutes remaining4 OF 15 QUESTIONS REMAIN1 PoirQuestion 7The G base of a DNA molecule has the molecular formula C₁0H12O6N5P. Which two elements in the given formula exhibit showsimilar chemical and physical properties?A) N and PO and PN and O
Answers
Answer
A) N and P
Explanation
Nitrogen and phosphorous are part of the same 'family' on the periodic table, group 15, also called the nitrogen group. Since phosphorus is just below nitrogen, we can expect nitrogen and phosphorus to have some similar properties. They are non-metals and have similar properties when making compounds.
MgSO4(aq) + 2NaOH(aq) Mg(OH)2(s) + Na2SO4(aq) i. Write the complete ionic equation from the balanced equation. (3 points) Please help!
Answers
Answer:
Mg²⁺(aq) + SO₄²⁻(aq) + 2Na⁺(aq) + 2OH⁻(aq) → Mg²⁺(aq) + 2OH⁻(s) + 2Na⁺(aq) + SO₄²⁻(aq)
Explanation:
The complete ionic equation is the chemical equation where the chemical species in the aqueous phase (aq) are written as ions.
In the reaction:
MgSO₄(aq) + 2NaOH(aq) → Mg(OH)₂(s) + Na₂SO₄(aq)
MgSO₄ dissociates in Mg²⁺ and SO₄²⁻, NaOH in Na⁺ and OH⁻, Mg(OH)₂ doesn't dissociate because is as solid and NaSO₄ dissociates in Na⁺ and SO₄²⁻ ions.
That means the complete ionic equation is:
Mg²⁺(aq) + SO₄²⁻(aq) + 2Na⁺(aq) + 2OH⁻(aq) → Mg²⁺(aq) + 2OH⁻(s) + 2Na⁺(aq) + SO₄²⁻(aq)Which statement about the quantum mechanical solution for the hydrogen atom is
correct?

Answers
The ml quantum number can be equal to 0 is the correct statement about the quantum mechanical solution for the hydrogen atom.
The lone electron in hydrogen can be found in an orbital in the ground state with the magnetic quantum number ml = 0, which is 1s.The same goes for this one electron, which can be excited to higher energy levels like 2p, 3p, and 3d, among others.Higher np sub-levels of the hydrogen atom's magnetic quantum numbers are -1, 0 and 1, respectively.The magnetic quantum numbers for the nd sub-levels are -2, -1, 0, 1, and 2. The information presented above shows that the magnetic quantum number for a hydrogen atom, ml, is not necessarily positive.For more information on quantum number kindly visit to
https://brainly.com/question/16977590
#SPJ1
what is the formula for selenium tetrafluoride
Answers
Answer:
SeF4
Explanation:
SeF4
Answer:
The anwser is SeF4
What are the terms that descripe these values for the element oxygen?

Answers
Answer:
16 is the mass number. 8 is the atomic number.
Population size can be used to describe ________ populations, not just ________.
It shows that the number of individuals in the population will ________ until the carrying capacity of the environment is reached.
Ecological models are _________ because they help scientists ________ what will occur in real life.
Explain what happened to the carrying capacity of reindeer on St. Matthew Island over time. ________
In ________ times, populations do well, and the number of individuals _________ rapidly. A period of rapid population ________ often follows, followed again by a ________ in numbers.
Answers
Population size can be used to describe all organism populations, not just animal populations.
It shows that the number of individuals in the population will increase until the carrying capacity of the environment is reached.
Ecological models are useful because they help scientists predict what will occur in real life.
What does population and population size means?The communities are made up of different species' populations. A population in biology is a group of organisms of the same species that live in the same area. Natural selection and evolution occur at the population level. The size and rate of growth of a population are frequently used as indicators of its health.
The number of people in a population is referred to as its population size. A population of insects, for example, could include 100 or more individual insects. The size of a population influences whether a species survives or goes extinct. In general, very small populations are most vulnerable to extinction. The size of a population, however, may be less important than its density.
Read more about Population size
brainly.com/question/25630111
#SPJ1
What elements ( ingredients) is made of?
A . Hydrogen
B . oxygen
C . Nitrogen
D . Carbon

Answers
Answer:
Two Hydrogen and Two Oxygen
Explanation: