What will determine the number of moles in a sample
Answers
Answer:
weigh it and divide the weight by the molecular weight. :) good luck!!
Explanation:
Answer:
TO FIND THE NUMBER OF MOLES IN A SAMPLE, SIMPLY WEIGHT IT AND DIVIDE THE WEIGHT BY THE MOLECULAR WEIGHT. THE QUOTIENT IS EQUAL TO THE NUMBER OF MOLES.
Related Questions
How long does S waves travel through earths crust
Answers
Answer:
For the distance range 50 to 500 km, the S-waves travel about 3.45 km/s and the P-waves around 8 km/s.
IF HELPED MARK AS BRAINLIEST
Answer:
For the distance range 50 to 500 km, the S-waves travel about 3.45 km/s and the P-waves around 8 km/s.
What are the most common metric units used in science?

Answers
Answer its a
Explanation:
at show clearly how bonding occured in (cao)
Calcium
oxide
Answers
Answer:
They both combine with The help of valency, which is the combining capacity of elements
Explanation:
I find it easier to understand valency as part of the Bhor’s model of an atom.
In his model, it was stated that electrons are spread around the nucleus in such a way that there are rings around the model. Each ring has a n value. So, k has 1, l has 2, m has 3 and n has 4...
what you need to know to find the number of electrons in each ring is to solve the formula 2n squared
Then, the valency is the 8 minus the number of electrons in the outermost shell. Remember that 8 is max number I;outer most shell. If you try it out for oxygen, you will get the value of 2 remaining, which can be gained if combined with calcium.
Hope it helps
Which of the following has the smallest mass? A. An electron B. An atomic nucleus C. A proton D. A neutron
Answers
Answer: A. An electron
Explanation:
Answer:
an electron
Explanation:
The electron has the lowest mass out of them all as the mass of electron is so little that it is neglected while calculating atomic mass which is proton+electron. the mass of electron is 10 to the power -30 kg
Define [Fluid compressibility, Solution-gas/liquid ratio, Fluid FVF, Fluid densities, and Fluid viscosities], write their equations, symbols, units \& correlations. (25-points)
Answers
1. Fluid compressibility (C): Fluid compressibility refers to the measure of how much a fluid's volume changes in response to a change in pressure.
2. Solution-gas/liquid ratio (SGLR): The solution-gas/liquid ratio represents the volume of gas dissolved in a given volume of liquid at a specific pressure and temperature.
3. Fluid formation volume factor (FVF): The fluid formation volume factor represents the ratio of the volume of a fluid at reservoir conditions (pressure and temperature) to its volume at surface conditions.
4. Fluid densities (ρ): Fluid densities refer to the mass per unit volume of a fluid.
5. Fluid viscosities (μ): Fluid viscosities represent the measure of a fluid's resistance to flow.
1. Equation: C = -1/V * dV/dP
Symbol: C
Unit: 1/Pascal (Pa^-1)
Correlation: The compressibility of fluids can vary depending on the fluid type. For ideal gases, the compressibility is inversely proportional to pressure.
2.Equation: SGLR = V_gas / V_liquid
Symbol: SGLR
Unit: Volumetric ratio (e.g., scf/bbl)
Correlation: The solution-gas/liquid ratio is influenced by the pressure and temperature conditions, as well as the composition of the fluid.
3. Equation: FVF = V_reservoir / V_surface
Symbol: FVF
Unit: Volumetric ratio (e.g., bbl/STB)
Correlation: The fluid formation volume factor depends on the composition and properties of the fluid, as well as the reservoir conditions.
4. Equation: ρ = m / V
Symbol: ρ
Unit: Mass per unit volume (e.g., kg/m^3)
Correlation: Fluid densities can vary depending on the type and composition of the fluid. For example, water has a density of approximately 1000 kg/m^3.
5. Equation: No single equation; viscosity is measured experimentally using viscometers.
Symbol: μ
Unit: Pascal-second (Pa·s) or centipoise (cP)
Correlation: The viscosity of a fluid is influenced by temperature and pressure. Different fluids exhibit different viscosities, ranging from low-viscosity fluids like water to high-viscosity fluids like heavy oil.
To know more about Fluid formation volume factor (FVF)
https://brainly.com/question/31458735
#SPJ11
3-methyl-1-butanol (also called isoamyl alcohol or isopentyl alcohol) was mixed with an excess of acetic acid (ethanoic acid is its systematic name) and a trace of sulfuric acid (which serves as a catalyst). This reaction is an equilibrium reaction, so it is expected that not all the starting materials will be consumed. The equilibrium should lie quite far to the right due to the excess of acetic acid used, but not completely.
After an appropriate length of time, isolation of the desired product from the reaction mixture was begun by adding a volume of 5% aqueous sodium bicarbonate (NaHCO3 has an effective pKa of 7) roughly equal to the volume of the reaction mixture. Bubbling occurred and a mixture consisting of two layers resulted—a basic aqueous layer and an organic layer.
The layers were separated, and the aqueous layer was removed.
The addition of aqueous sodium bicarbonate to the layer of organic materials and separation of the layers was repeated twice. Each time the predominantly aqueous layers were removed, they were combined in the same collection flask.
The organic layer that remained after the three bicarbonate extractions were dried and then subjected to distillation to obtain a pure sample of 3-methylbutyl ethanoate (isoamyl acetate).
List all the chemical species likely to be present at the end of the reaction but before adding aqueous NaHCO3. Note that the H2SO4 was not consumed (since it is a catalyst).
Answers
At the end of the reaction before adding aqueous \(NaHCO_3\), the following chemical species are likely to be present:
3-methyl-1-butanol (isoamyl alcohol or isopentyl alcohol).Acetic acid (ethanoic acid).3-methylbutyl ethanoate (isoamyl acetate).Water.Sulfuric acid (catalyst).A Lewis structure, also known as a Lewis dot diagram, is a way to represent the chemical bonding in a molecule. It uses dots (also called electron dots or Lewis dots) to show the valence electrons on an atom, and lines to show the bonds between atoms. The goal of drawing a Lewis structure is to use the valence electrons of the atoms in a molecule to form the most stable arrangement of atoms, that is to say, to achieve the octet rule where each atom has 8 valence electrons in its outermost shell.
Learn more about chemical, here https://brainly.com/question/13145357
#SPJ4
A 0.010 M aqueous solution of a weak acid HA has a pH of 5.0. What is the degree of ionization of HA in the solution?
a. 1 %
b. 0.01 %
c. 10 %
d. 0.1 %
e. 0.001 %
Answers
The pH of the solution is 5.0, which means the [H3O+] concentration is 10^-5 M. Since HA is a weak acid, it can dissociate in water as shown below:
HA + H2O ⇌ H3O+ + A-
Let x be the degree of ionization of HA. The concentration of H3O+ ions in the solution is the same as the concentration of A- ions formed by the dissociation of HA. Therefore, we can write the equilibrium expression as:
Ka = [H3O+][A-]/[HA]
Substituting the known values, we get:
4.0 × 10^-10 = (x)(x)/(0.010 - x)
Solving for x gives us x = 1.0 × 10^-3, which is 0.1%. Therefore, the degree of ionization of HA in the solution is 0.1%. The correct answer is (d).
To know more about pH please visit:
https://brainly.com/question/15289741
#SPJ11
HELP ME PLEASE!!
Density (D) is defined by mass/volume .
Which item increases as heat is applied?
D
m
V
Answers
Answer:
volume
Explanation:
things expand when heat is applied taking up more space hence volume
how is melting similar to freezing
Answers
Answer:
no similarities but if that's so it's temperature
What is the purpose of the cell wall?
Answers
The purpose of cell wall is to protect the cell and to determine the cell's shape.
d. 2 5. How many moles of HCl are needed to produce 25 moles of KCl in the presence of excess potassium permanganate? a. 16 b. 32 c. 5 d. 200
Answers
Answer:
d. 200.
Explanation:
The reaction is:
2KMnO4 + 16HCl ----> 2KCl + 2MnCl2 + 8H2O + 5Cl2
So 2 moles of KCL are produced form 16 moles of HCL
So 25 moles KCL are made from 25 * (16/2) HCl.
= 25 * 8
= 200 moles. HCl.
(Help Me Please!)
A student collected the data shown above. Row A may represent error
B
C
D
E
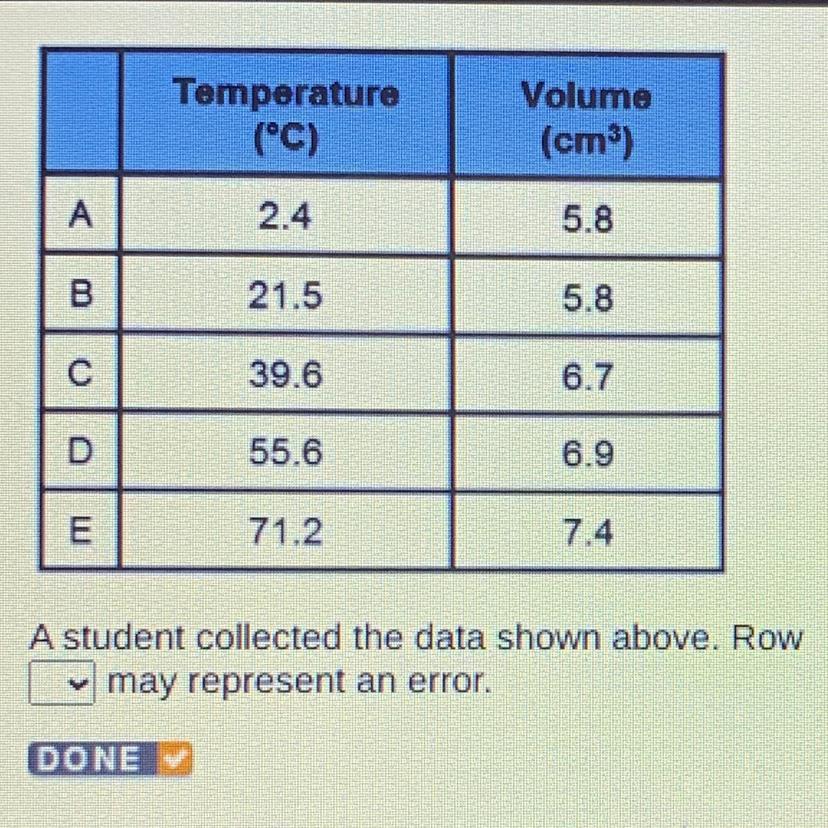
Answers
It’s the biggest diff of temp without volume
the rate constant of a dimerization reaction is 0.014 l/moles. what will be the concentration of its reactant in molar after 1.3 hours if the initial concentration is 0.36 m? report a numerical value with 3 decimal places, without units.
Answers
The concentration of reactant after 1.3 hours is 0.353 m when the rate constant of a dimerization reaction is 0.014 l/moles.
The dimerization reaction follows a second-order rate law, so the rate equation can be written as k[A]^2, where k is the rate constant and [A] is the reactant concentration. Using the given rate constant of 0.014 l/moles and initial concentration of 0.36 m, we can use the integrated rate law to find the concentration of reactant after 1.3 hours.
ln([A]t/[A]0) = -kt
ln([A]t/0.36) = -(0.014 l/moles)(1.3 hours)
ln([A]t/0.36) = -0.0182
[A]t = 0.36e^(-0.0182)
[A]t = 0.353 moles/l
Rounding to 3 decimal places, the concentration of reactant after 1.3 hours is 0.353 m.
To know more about rate constant visit
https://brainly.com/question/14951855
#SPJ11
The diagram below shows the different phase transitions that occur in matter.
Solid
3
Liquid
16
Gas
Which arrow represents the transition in which dew is formed?
1
O2
4
6
Answers
Answer:
C.4
Explanation:
The arrow 4 represents the transition in which dew is formed.
What is Dew?This is formed as a result of condensation which takes place in the morning or evening and is usually seen on exposed surfaces as water droplets.
The air particles in the form of vapor under low temperatures results in the formation of the substance. The arrow 4 thereby depicts it and makes it the most appropriate choice.,
Read more about Dew here https://brainly.com/question/2696211
In which reaction is a small molecule formed from the atoms removed from a single reactant molecule?
Answers
Explanation:
During condensation reaction, two molecules combine to form a single molecule with the loss of a small molecule; in dehydration reaction, this lost molecule is water.
The reaction is a small molecule formed from the atoms removed from a single reactant molecule this reaction is knowing as condensation reaction.
How is the condensation process?Essentially, this is the reverse process of vaporization. In order for water vapor to undergo the process of condensation, which is the change from a gaseous state to a liquid state, it is necessary for a reduction in its temperature to occur until it reaches the dew point or an increase in the pressure to which it is subjected.
In this case the condensation reaction are when two molecules combine to form a single molecule with the loss of a small molecule, in dehydration reaction, this lost molecule is water.
See more about condensation at brainly.com/question/15563071
#SPJ2
how did j.j. thomson discover that there are atoms (chemical elements) that have the same properties but have different masses? what are these called? what was his view of the atom at that time?
Answers
J.J. Thomson discovered isotopes, which are atoms of the same element with different masses, by studying cathode rays in a mass spectrometer.
Thomson's mass spectrometry experiments showed that neon gas contained two types of atoms, which he called isotopes, with different masses but identical chemical properties. His view of the atom at the time was that it was indivisible and consisted of a positively charged "pudding" with negatively charged electrons embedded throughout it.
Thomson named these different types of atoms "isotopes" from the Greek words for "same place," because they occupy the same place on the periodic table and have the same chemical properties, but differ in their atomic masses.
To know more about the atoms, here
brainly.com/question/18747362
#SPJ4
Which of these pairs of elements is most likely to be part of a polyatomic ion?
I and I?
S and O?
or K and F.
Answers
The pairs of elements that is most likely to be part of a polyatomic ion is option B: S and O.
What is polyatomic ion?An ion made up of two or more atoms that really combine covalently is referred to as a polyatomic ion. This implies that only non-metals can link covalently with one another since a covalent bond is created when electrons are shared.
S and O atoms, for instance, have a covalent bond and frequently combine to produce polyatomic ions. K and F, Li and I, Mg and Br, on the other hand, are both metals and non-metals. Therefore, because the metal will donate its valence electrons to the non-metal, they will always establish an ionic bond.
As a result, we can draw the conclusion that, among the possibilities provided, S and O pairs of elements are most likely to make up a polyatomic ion.
Learn more about polyatomic ion from
https://brainly.com/question/13659069
#SPJ1
what is the h+ oh- and poh of solution with ph=3.67
Answers
Calculate the volume in mL of 53.2 g of .251M solution of HCl.
Show work please
Answers
Answer:
5.813 mL
Explanation:
You need to use the formula : vol = mass / molar mass x molarity
the molar mass of HCl is 36.46 and the problem gives you the rest of the equation
0.251M x 36.46 = 9.151
53.2g / 9.151 = 5.813 mL
*I don't know some of the units so that's why some of them are blank sorry
*I'm also sorry if this is wrong but it's what I did so..
SEP Use Mat Calculate the electronegativity differences and determine the
polarity for the bonds formed by the following pairs of atoms: K and F atoms and
N and O atom
Answers
This problem is asking for the electronegativity differences and polarity for the bonds formed between and K and F, and N and O. At the end, the results are 3.16 and ionic, and 0.40 and covalent, respectively.
Types of bondsIn chemistry, chemical bonds are formed when elements either share or gain electrons. Once they form the bond, one can tell much of the properties of the resulting compound based on the type of bond formed.
In such a way, one can evidence de formation of covalent and ionic bonds, the former exhibited when the electronegativity difference is between 0 and about 1.6 and the former beyond 1.7.
ElectronegativityIn chemistry, electronegativity is the tendency of an atom to attract electrons in a molecule, thus, we have the following for K, F, N and O as required: 0.82, 3.98, 3.04 and 3.44, respectively.
In such a way, one can calculate the difference electronegativity for the pairs KF and NO as shown below:
K-F =3.98 - 0.82 = 3.16
N-O = 3.44 - 3.04 = 0.40
Hence, the K-F bond turns out ionic whereas the N-O, covalent.
Learn more about electronegativity: https://brainly.com/question/2060520
Show work please ?...........

Answers
Answer:
NO
Explanation:
NO NO NO
i am way to much of an idiot for this (hope i made you laugh (and mad cuz i did not answer) :3)
Read the following excerpt and answer the question:
Scientists often refer to mushrooms as the "dark matter of biology" because we don't know much about them. Mushrooms are not plants
because they have no chlorophyll and cannot make their own food. They are actually fungi and absorb nutrients from their surrounding
environments, and they can even thrive at the bottom of the ocean or in the middle of a desert!
Use your own words to summarize why mushrooms and plants differ in their needs to use radiant energy from the sun.
Answers
Answer:
\/
Explanation:
Plants rely directly on the energy from the sun. The chloroplast create glucose from the sun rays through a process known as photosynthesis. Mushrooms, on the other hand, do not have the ability to use the sun's rays to create food, so they instead gather their energy from the nutrients from the soil or decaying things, such as plants, that once used the sun's radiation as sustenance. So basically mushrooms use the radiant energy from the sun indirectly and plants use it directly
Which of the following is NOT a fundamental subatomic unit of an element?
O Ionic
O Polar
O Covalent
O Nucleus
Answers
The rest of the answers are types of chemical bonds
The model of the atom changed as new evidence was discovered. The plum pudding model suggested that the atom was a ball of positive charge with electrons embedded in it. Evidence from the alpha particle scattering experiment led to a change in the model of the atom from the plum pudding model. Explain how. PLS HELP RN :)
Answers
Answer:
so since the model atom was changed as new evidence, and the plum pudding model was a ball of positive charge, the alpha particle scattering which broke up the atom
A chemist has a block of copper metal (density is 8.96 g/mL). The block weighs 2.30 g. What is the volume of the copper block in mL
Answers
Thus, the answer is the volume of the copper block in mL is 0.256696 mL.
A chemist has a block of copper metal (density is 8.96 g/mL). The block weighs 2.30 g. What is the volume of the copper block in mL?
The density is given as density = 8.96 g/mL
The weight of the block of copper metal is given as weight = 2.30 g
We know that density = weight/volume,
rearranging this we get;
volume = weight/density
Thus, substituting the values given, we get;
volume = 2.30 g / 8.96 g/mL
The volume of the copper block in mL is given as;
volume = 0.256696 mL
Therefore, the volume of the copper block in mL is 0.256696 mL or about 0.257 mL.
to know more about volume visit:
https://brainly.com/question/28058531
#SPJ11
The pH of the ocean is around 8.1, is the ocean considered a
buffer? Why or Why not?
Answers
Yes, the sea is considered a buffer.
A buffer is a solution that resists pH changes when acids or bases are added. The buffering capacity of the ocean allows it to maintain a relatively stable pH even when acids and bases are added.
The ocean's buffering capacity is primarily due to the presence of dissolved compounds such as bicarbonate (HCO3-) and carbonate (CO32-). These compounds act as both weak acids and bases, accepting and releasing hydrogen ions (H+) to maintain pH balance. When carbon dioxide (CO2) in the atmosphere dissolves in seawater, carbonic acid (H2CO3) is produced and decomposed into bicarbonate ions and hydrogen ions.
This transformation helps prevent a rapid drop in pH as excess hydrogen ions combine with carbonate ions to form bicarbonate ions, which can reduce overall acidity.
When alkali such as hydroxide ions (OH-) is added to the ocean, excess hydroxide ions combine with hydrogen ions to form water molecules, reducing alkalinity.
The presence of these dissolved compounds and their interconversion reactions stabilize the pH of the ocean, making it less susceptible to rapid changes in acidity or alkalinity. This buffering capacity is essential for the survival and maintenance of marine life, as many organisms are sensitive to changes in pH.
To know more about PH refer to this link
https://brainly.com/question/12609985
Calculate the amounts of p-acetamidophenol (in grams) and bromoethane (in milli- liters) that are used in this reaction. 5. What is activated carbon? What is it used for, either in the lab or other applications?
Answers
the amount of bromoethane (in milli- liters) that are used in this reaction is 74.13ml.
Mol wt of P-Acetamido phenol = 151.16. Mol wt of Bromoethane = 108.97. Mol wt of Acetophenetidin (product) = 179.22. Paraacetamido phenol, mol wt=151.16. Bromoethane= 108.97. By the law of mass action, 151.16 g P-Acetamido phenol will react 108.97g Bromoethane to get 179.22 g Acetophenetidin (product) and 80.91 g Hydrobromic acid as a bye product. Since the density of Bromoethane = 1.47g/cc, the quantity of Bromoethane in milliliters = 108.97/1.47 = 74.13 ml. Activated carbon, also known as activated charcoal, is a form of carbon that has been processed to have small, low-volume pores that increase the surface area available for adsorption or chemical reactions. Activated carbon is used for a wide range of industrial and environmental applications, including purifying air and water, removing impurities from liquids and gases, and recovering valuable chemicals. In the laboratory, activated carbon is commonly used to purify organic compounds, remove dissolved gases from liquids, and purify water.
Learn more about chemical reactions here:
https://brainly.com/question/29762834
#SPJ4
why elements with more than 20 protons always have a more neutrons than protons?
Answers
Answer:
Elements that have atomic numbers from 20 to 83 are heavy elements, therefore the ratio is different. The ratio is 1.5:1, the reason for this difference is because of the repulsive force between protons: the stronger the repulsion force, the more neutrons are needed to stabilize the nuclei.
how many minutes will it take to plate out 2.19 g of chromium metal from a solution of cr3 using a current of 55.2 amps in an electrolyte cell? how many minutes will it take to plate out 2.19 g of chromium metal from a solution of using a current of 55.2 amps in an electrolyte cell? 1.23 73.7 3.68 11.0 221
Answers
It will take approximately 3.5 minutes to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 55.2 amps in an electrolyte cell.
To calculate the time it will take to plate out 2.19 g of chromium metal using a current of 55.2 amps in an electrolyte cell, we can use Faraday's law of electrolysis.
First, we need to calculate the number of moles of Cr3+ ions that will be reduced to form the chromium metal. The molar mass of Cr is 52 g/mol, so 2.19 g of Cr is equivalent to 0.0421 moles (2.19 g / 52 g/mol).
Next, we need to determine the number of electrons required for the reduction of each Cr3+ ion to form Cr. From the half-reaction equation for the reduction of Cr3+ to Cr, we know that 3 electrons are required.
Using Faraday's law, we can calculate the total charge (Q) required to reduce 0.0421 moles of Cr3+ ions to form Cr:
Q = nF
where n is the number of moles of Cr3+ ions (0.0421 mol) and F is the Faraday constant (96,485 C/mol).
Q = 0.0421 mol x 3 x 96,485 C/mol = 11,726 C
Finally, we can calculate the time (t) required to plate out 2.19 g of chromium metal using a current of 55.2 amps:
t = Q / I
where I is the current (55.2 A).
t = 11,726 C / 55.2 A = 212.5 seconds or approximately 3.5 minutes
Therefore, it will take approximately 3.5 minutes to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 55.2 amps in an electrolyte cell.
Visit here to learn more about Faraday's law : https://brainly.com/question/1640558
#SPJ11
Atmospheric pressure is the sum of the pressure created by all gases in the
atmosphere. How does the atmospheric pressure change as you move
from places of high elevation to low elevation?
Your answer
Answers
Answer:
high less air cause there is no trees and lower is the same
Explanation: