Answers
Answer:
movement of charged particles through the wire .
Explanation:
When electricity is passed through the wire of electromagnet , moving electrons of the wire produces magnetic field . This magnetic field in increased due to high permeability of soft iron of the electromagnet . It is this magnetic field which creates magnetic force .
Related Questions
can I get some urgent help please?

Answers
Answer:
hi here goes your answer
Explanation:
iv. The lower the PH, the weaker the base
Choose the one statement that is true of the noble gases.A.They are very reactive.B.Their valence shells are full of electrons.C.They are unstable.D.They are liquids at room temperature.
Answers
B. Their valence shell are full of electrons
That is the main characteristic of noble gases, and it is because of this that they are very stable, very unreactive and they have very low condensation temperature.
How many atoms of oxygen are on the reactants side of this balanced equation?
2AgNO3 (aq)+ Cu(s) —Cu(NO3)2 (aq)+ 2Ag(s)
12
2
3
6
Answers
Answer:
Explanation:
I tried to figure it out but my head wasn’t working sorry
Which of these molecules is polar?
A. O₂
B. N2
C. BH3
D. NO
Answers
Explanation:
Then there is no net charge separation. a BH3 b CHCl3 c C2H2 d NH3. Optionally the ... Oxygen O2 c. ... BH3 2. Com Bh3 Polar Or Nonpolar In the case of non polar molecules dispersion forces or London forces are present between them.
The polar molecules is NO.
What is polar molecule?A polar molecule is one that has one end that is slightly positive and the other end that is slightly negative. Ionic or polar covalent bonds can form in polar molecule. A dipole is a molecule that has two poles. The dipole moment is the outcome of measuring the amount of polarity in a molecule.
NO molecule, In the periodic table, nitrogen is to the right of oxygen. Nitrogen has a lower electronegative potential than oxygen. All N-O bonds are polar, with the oxygen atom having a higher electron density. The uneven distribution of electrons in the NO molecule is quite minor. When a molecule is non-polar, the electrons are shared evenly, resulting in a non-polar bond, or the polar bonds are symmetric. So, oxygen, nitrogen and boron trihydride molecules are non-polar molecule.
Hence the correct option is D.
To know more about polar molecule, here,
https://brainly.com/question/11405437
#SPJ2
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestWhat tiles should I put in the box?

Answers
Answer:
true&answe
Explanation:
I WILL GIVE 35 POINTS TO THOSE WHO ANSWER THIS QUESTION RIGHT NOOOO SCAMS PLEASE

Answers
The solution has a molarity of 0.0924 M.
What is molarity, for instance?The number of moles of solute per litre of solution is known as molarity.. For instance, water is both the solution and the solute when table salt is dissolved in it. Each mole of sodium chloride weighs 58.44 grammes. 58.44 grammes of sodium chloride are dissolved in one litre of water to produce one molar solution, or 1M.
Moles of solute per litre of solution is known as molarity (M).
Given: moles of NH3 = 0.355, volume of solution = 3.84 L
Molarity = 0.355 moles / 3.84 L = 0.0924 M
Therefore, the molarity of the solution is 0.0924 M.
To know more about molarity visit:-
https://brainly.com/question/8732513
#SPJ1
Preliminary preformulation studies for a new candidate drug molecule does not include one of these
A. Identity
B. Formula and weight
C. pharmacological activity
D. Pilot scale up
Answers
Answer:
Pilot scale up
Explanation:
Preformulation studies are carried out on candidate drug molecules that show sufficient pharmacological promise in animal model(pharma approach).
It involves preliminary study of the properties of a drug which is considered a potentially active ingredient against a particular disease condition.
Scale-up is the term used to refer to the increase in the batch size of a product. This is only done after a drug has been proven successful against the target disease after extensive pilot studies.
Scale-up is the last operation carried out when a drug has passed through all stages. It is not included in preliminary preformulation studies
a tire will burst if the air inside it reaches a pressure greater than 1.4 x 10^3 kpa. at what temperature will the tire burst if it has a volume of 30L and contains 2.5 mol of air? assume that the air behaves as an ideal gas. assuming that these values are representative, do you need to worry about your car tire bursting from overheating of they are in good condition?
Answers
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
To determine the temperature at which the tire will burst, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for temperature, we have:
T = PV / (nR)
Given that the pressure threshold for bursting is 1.4 x 10^3 kPa, the volume is 30 L, and the number of moles of air is 2.5 mol, we can substitute these values along with the ideal gas constant R = 8.314 J/(mol K) into the equation.
T = (1.4 x 10^3 kPa) * (30 L) / (2.5 mol * 8.314 J/(mol K))
Converting kPa to Pa and L to m^3, and simplifying the equation, we find:
T ≈ 20,993 K
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
For more question on temperatures
https://brainly.com/question/4735135
#SPJ8
An electrolysis reaction is
A) spontaneous
B) exothermic
C) non-spontaneous
D) hydrophobic
Answers
Answer:
it's non-spontaneous
Explanation:
I hope it helps you
Silver has a density of 10.5 g/cm³, and gold has a density of 19.3 g/cm³. Which would have a greater mass, 5 cm³ of silver or 5 cm³ of gold?
Answers
Gold have a greater mass
Further explanationDensity is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
\(\large {\boxed {\bold {\rho ~ = ~ \frac {m} {V}}}}\)
ρ = density
m = mass
v = volume
mass of Silver :\(\tt mass=\rho\times V\\\\mass=10.5\times 5=52.5~g\)
mass of Gold :\(\tt mass=\rho\times V\\\\mass=19.3\times 5=96.5~g\)
Salts when dissolved in water releases
Answers
Water molecules pull the sodium and chloride ions apart, breaking the ionic bond that help them together.After the salt compounds are pulled apart ,the sodium and chloride atoms are surrounded by water molecules,as this diagram shows.Once this happens,the salt is desolved,resulting in a homogeneous solution
combustion always result in to formation of water. what other type of reactions may result into formation of water? examples of these reactions
Answers
As combustion always result into the formation of water, the other type of reactions that may result into formation of water are Acid-Base Neutralization Reactions and Hydrogen and Oxygen Reaction.
Acid-Base Neutralization Reactions:
A neutralisation reaction is a chemical process in which an acid and a base combine to produce salt and water as the end products.
H⁺ ions and OH⁻ ions combine to generate water during a neutralisation reaction. Acid-base neutralisation is the most common type of neutralisation reaction.
Example: Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H₂O
Hydrogen and Oxygen Reaction:
Water vapour is created when hydrogen gas (H₂) and oxygen gas (O₂) are combined directly. This reaction produces a lot of heat and releases a lot of energy.
Example: 2 H₂ + O₂ → 2 H₂O
Learn more about reactions:
https://brainly.com/question/25769000
Hello I need help on the question 3 for chemistry. Also-this is a worksheet for practice! Thank you!

Answers
1) List the known and unknown quantities.
Sample: carbon dioxide (CO2).
Volume: 250 mL.
Pressure: 1.0 atm.
Temperature: 22 ºC.
Ideal gas constant:
2) Set the equation
\(PV=nRT\)3) Converting units.
3.1-Convert ºC to K.
\(K=ºC+273.15\)\(K=22\text{ }ºC+273.15=295.15\text{ }K\)3.2-Convert mL to L
1000 mL = 1 L
\(L=250\text{ }mL*\frac{1\text{ }L}{1000\text{ }mL}=0.250\text{ }L\)4) Plug in the known quantities
Of the following compounds, which has the strongest dipole?
Select one:
O a. CCl4
O b. SiO2
OC. PC13
O d. H₂O
O e. Brci
Answers
Answer: D.) H₂O
Explanation:
It can't be A because the molecular geometry is tetrahedral and all the bonding atoms are the same and evenly dispersed, cancels out all dipoles.Can't be B because the molecular geometry is linear and bonding atoms are the same. Therefore the bond dipole cancel out.Can't be C because the molecular geometry is trigonal planar and all the bonding atoms are the same. Therefore all the bond dipoles cancel out.Can't be E because while there is a dipole, it is not as large as the dipole created by an Oxygen Hydrogen bond like the one found in H₂O.In terms of energy, when atoms chemically bond to form a stable compound:_____.
A. Energy is released.
B. Energy can either be released or consumed depending upon the bond formed.
C. Energy is transferred from one atom to another.
D. Energy is consumed.
Answers
Answer:
A. Energy is released
Explanation:
Energy is only released when chemical bonds are formed.
A sample of water goes through a temperature change of -74.14 °C while releasing 578.7 joules of heat. The specific heat capacity of water is 4.184 J/(g·°C). What is the mass of this sample? grams (Report your answer using 4 significant figures).
Answers
Answer:
\( \boxed{ \sf \: m = 1.866 \: grams}\)
Explanation:
Given:
Change in temperature ∆T= -74.14°C
Heat(Energy) released Q= 578.7 joules
Specific heat capacity s = 4.184 J/(g·°C)
To find:
Mass of the sample (m)=?
Solution:
\( \sf s= \frac{Q}{m \cdot \Delta T} \\ \sf \: Substituting \: the \: given \: parameters \\ \sf 4.184 = \frac{578.7}{m \times ( - 74.14)} \\ \sf m = \frac{578.7}{4.184 \times ( - 74.14)} \\ \sf m = \frac{578.7}{310.20176} \\ { \sf m = 1.8655 \approx1.866 \: grams}\)
Since, we need the answer in 4 significant figures,
\( \boxed{ \sf \: m = 1.866 \: grams}\)
Learn more about specific heat here
brainly.com/question/27365354
Thanks for joining brainly community!
reaction will be spontaneous at all temperatures if _____
Answers
If a reaction has a negative ΔG and a positive ΔS, the reaction will be spontaneous at all temperatures.
If a reaction is spontaneous at all temperatures, it implies that the reaction will occur without the need for any external intervention, such as the addition of energy. For a reaction to be spontaneous, it must satisfy the criteria of thermodynamic favorability, which is determined by the change in Gibbs free energy (ΔG) associated with the reaction.
The relationship between ΔG, temperature (T), and the equilibrium constant (K) of a reaction is described by the equation ΔG = ΔH - TΔS, where ΔH is the change in enthalpy and ΔS is the change in entropy.
To ensure spontaneity at all temperatures, two conditions must be met:
ΔG must be negative: A negative ΔG indicates a thermodynamically favorable reaction, meaning the products have a lower Gibbs free energy than the reactants. If ΔG is negative, the reaction will proceed spontaneously in the forward direction.
ΔS must be positive: A positive ΔS signifies an increase in the overall entropy of the system. Higher entropy means more disorder, and spontaneous reactions often involve an increase in randomness. When ΔS is positive, it can compensate for the enthalpic term, ΔH, allowing the reaction to proceed spontaneously.
For more such questions on spontaneous visit:
https://brainly.com/question/30127476
#SPJ8
To identify a diatomic gas ( X2 ), a researcher carried out the following experiment: She weighed an empty 6.4- L bulb, then filled it with the gas at 1.30 atm and 27.0 ∘C and weighed it again. The difference in mass was 9.5 g . Identify the gas.
Answers
The diatomic gas ( X2 ) is N₂ dinitrogen.
Dinitrogen is a chemical compound fashioned from the covalent bonding of two nitrogen atoms. it's far a colorless, odorless gas at room temperature and pressure, which makes up about seventy-eight % of the Earth's environment.
Diatomic gas is a chemical compound formed from the covalent bonding of two nitrogen atoms. it's miles drab, odorless gasoline at room temperature and stress, which makes up about seventy eight % of the Earth's surroundings.
Volume = 6.4 L
Pressure = 1.3 atm
Temperature = 25 C = 298 K
R = 0.08206 L.atm/mol.K
P * V = n * R * T
1.3 atm * 6.4 L = n * 0.08206 L.atm/mol.K * 298 K
n = 0.34 moles
difference of mass is the mass of gas = 9.5 g
Molar mass = Mass / No. of moles = 9.5 g / 0.34 moles = 27.9 g/mol
diatomic gas with molar mass 28 g/mol is N2
Hence the diatomic molecule is N2
Learn more about dinitrogen here:-https://brainly.com/question/11651796
#SPJ9
3- Calculate the mass of sodium chloride produced it :
Sodium bicarbonate is 12g
Hydrochloric acid is 5.58g
Water is 2.75g
Carbon Dioxide is 6.82g
Answers
The mass of sodium chloride produced by the chemical reaction between sodium bicarbonate and hydrochloric acid is 8.37g.
The balanced chemical equation for the reaction between sodium bicarbonate (NaHCO₃) and hydrochloric acid (HCl):
NaHCO₃ + HCl → NaCl + H₂O + CO₂
1 mole of sodium bicarbonate (NaHCO3) reacts with 1 mole of hydrochloric acid (HCl) to produce 1 mole of sodium chloride (NaCl), 1 mole of water (H₂O), and 1 mole of carbon dioxide (CO₂).
NaCl: Molar mass = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
The number of moles of sodium bicarbonate (NaHCO₃) and hydrochloric acid (HCl) using their respective masses:
Number of moles of NaHCO₃ = Mass of NaHCO₃ / Molar mass of NaHCO₃
Number of moles of NaHCO₃ = 12 g / (22.99 g/mol + 1.01 g/mol + 48.00 g/mol + 16.00 g/mol)
Number of moles of NaHCO₃ = 12 g / 84.00 g/mol ≈ 0.143 moles
Number of moles of HCl = Mass of HCl / Molar mass of HCl
Number of moles of HCl = 5.58 g / (1.01 g/mol + 35.45 g/mol)
Number of moles of HCl = 5.58 g / 36.46 g/mol ≈ 0.153 moles
Since the reaction stoichiometry is 1:1 between NaHCO₃ and NaCl, the number of moles of NaCl produced will be the same as the number of moles of NaHCO₃ reacted. Therefore, the mass of NaCl produced can be calculated as follows:
Mass of NaCl produced = Number of moles of NaCl × Molar mass of NaCl
Mass of NaCl produced = 0.143 moles × 58.44 g/mol
The Mass of NaCl produced ≈ 8.37 g
Hence, the mass of sodium chloride produced is approximately 8.37 grams.
Learn more about chemical reactions, here:
https://brainly.com/question/22817140
#SPJ1
According to the law of conservation of matter, we know that the total number of atoms does not change in a chemical reaction and thus mass is conserved. The reactant in this model is hydrogen peroxide. Hydrogen peroxide decomposes to produce water and oxygen gas. How many water molecules must be added to complete this model?
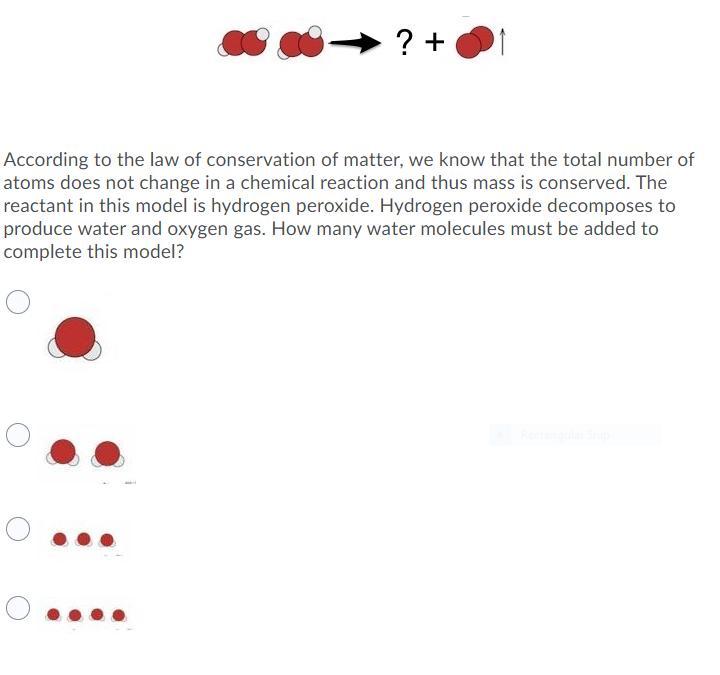
Answers
Answer:
the answer is A
Explanation:
what does the electronegravity of an atom indicate?
Answers
Answer:
The electronegativity of an atom indicates the ability of an atom to attract shared electrons in a covalent bond. The higher the value of the electronegativity, the more strongly that element attracts the shared electrons.
Hope this helps!!!
Electronegravity of an atom indicates The ability an atom or molecule has to attract electrons in the context of a chemical bond.
Approximately 50% of our bone is chemically calcium phosphate, Ca3(PO4)2
If an adult has 12 kg of bone, calculate the mass of calcium is present
Answers
A sample of gas occupies 7.80 liters at 425°C? What will be the volume of the gas at 35°C if the pressure does not change?
Answers
Answer:
How do amoeba respire.
how do plants respire.
Discuss the large-scale environmental impacts of soil pollution caused by industrial wastes.
Answers
Answer: Industrial processes including mining and manufacturing historically have been leading causes of soil pollution. Industrial areas typically have much higher levels of trace elements and organic contaminants. This is due to intentional and unintentional releases from industrial processes directly into the environment, including to the soil, adjacent water bodies, and the atmosphere.
Explanation:
Which of the following best defines crustal deformation? the constructive force of hot molten rock from the mantle that reaches Earth's surface, resulting in new landforms the outermost rocky layer of Earth the constructive force that moves sediments from one place and lays them to rest at another, forming landforms the constructive force that is the result of the edges of Earth's crust pushing and pulling against each other
Answers
The best definition of crustal deformation is that it is the constructive force that is the result of the edges of Earth's crust pushing and pulling against each other.
What is crustal deformation?Crustal deformation refers to the changes in the shape, position, and orientation of the Earth's crust due to the forces acting on it.
These forces can be compressional, tensional, or shear, and they cause the crust to buckle, fold, fault, and uplift.
Crustal deformation can result in the formation of new landforms, such as mountains, valleys, and plateaus, and can also cause earthquakes and volcanic eruptions.
Thus, the best definition of crustal deformation is that it is the constructive force that is the result of the edges of Earth's crust pushing and pulling against each other.
More on crustal formation can be found here: https://brainly.com/question/13490737
#SPJ1
Why KHPo4 ignore effective as a buffer but kh2po4 is not
Answers
KH2PO4 is a more suitable choice as a buffer because it has a greater buffering capacity due to the presence of the weak acid and its conjugate base.
KHPo4 is not considered an effective buffer compared to KH2PO4 due to its limited buffering capacity. The effectiveness of a buffer is determined by the concentration and dissociation properties of its conjugate acid-base pair.
KH2PO4 is a salt composed of the weak acid H2PO4- and its conjugate base HPO4^2-. In an aqueous solution, KH2PO4 can dissociate to release H+ ions from the H2PO4- component, which acts as a weak acid, and the HPO4^2- component can accept H+ ions, acting as a weak base. This allows KH2PO4 to effectively resist changes in pH when small amounts of acid or base are added to the solution.
On the other hand, KHPo4 consists of the strong acid H3PO4 and the weak base HPO4^2-. H3PO4 fully dissociates in water, providing a large concentration of H+ ions, making it difficult for the HPO4^2- to effectively act as a base and maintain pH stability.
Therefore, KH2PO4 is a more suitable choice as a buffer because it has a greater buffering capacity due to the presence of the weak acid and its conjugate base.
For more question on conjugate
https://brainly.com/question/14684465
#SPJ8
The actual density of pennies made before 1982 is about 8.8 g/mL. The actual density of pennies made after 1982 is about 7.2 g/mL. Compare your results with these accepted values. What could you have done differently to obtain results closer to the accepted values?
Answers
To obtain more accurate values of the densities of the pennies:
more trials can be conducted to reduces the effect of measurement errorsmore care should be taken in taken volume measurements by displacement of water to avoid splashing of water.What is density of a substance?The density of a substance is the ratio of the mass of the substance to the volume of that substance.
It is a measure of the compactness of the matter in a substance.
The densities of the pennies made before 1982 was 8.7 g/ml
The actual density of pennies made before 1982 is about 8.8 g/mL.
The densities of the pennies made after 1982 was 6.9 g/ml
The actual density of pennies made after 1982 is about 7.2 g/mL.
Due to several random and experimental errors, the values obtained from the experiment are not exactly the same with the accepted values.
To obtain more accurate values:
more trials can be conducted to reduces the effect of measurement errorsmore care should be taken in taken volume measurements by displacement of water to avoid splashing of water.In conclusion, the densities varied as as a result of random and experimental errors.
Learn more about density at: https://brainly.com/question/1354972
#SPJ1
Answer:
Prior to 1982, the densities of pennies were 8.7 g/ml.
The density of pre-1982 pennies is approximately 8.8 g/mL.
The densities of pennies produced after 1982 were 6.9 g/ml.
The density of pennies made after 1982 is approximately 7.2 g/mL.
The values obtained from the experiment do not exactly match the accepted values due to several random and experimental errors.
Explanation:
How many moles of aluminum ions al3+ are present in 0.42 mol of al2so43
Answers
There are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
To determine the number of moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3, we need to consider the stoichiometry of the compound.
The formula of aluminum sulfate (Al2(SO4)3) indicates that for every 1 mole of the compound, there are 2 moles of aluminum ions (Al3+). This means that the mole ratio of Al3+ to Al2(SO4)3 is 2:1.
Given that we have 0.42 mol of Al2(SO4)3, we can calculate the moles of Al3+ as follows:
Moles of Al3+ = 0.42 mol Al2(SO4)3 x (2 mol Al3+ / 1 mol Al2(SO4)3)
Moles of Al3+ = 0.42 mol Al2(SO4)3 x 2
Moles of Al3+ = 0.84 mol Al3+
Therefore, there are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
It's important to note that the stoichiometry of the compound determines the mole ratio between the different species involved in the chemical formula. In this case, the 2:1 ratio of Al3+ to Al2(SO4)3 allows us to determine the number of moles of Al3+ based on the given amount of Al2(SO4)3.
For more such question on aluminum visit:
https://brainly.com/question/30451292
#SPJ8
A sample of gas at 2815 torr is cooled from 150.0 C to 100.0 C. Assuming the volume is constant what is the pressure in atm of the gas at 100.0 C
Answers
A sample of gas at 2815 torr is cooled from 150.0 C to 100.0 C. Assuming the volume is constant, 2482.2torr is the pressure in atm of the gas at 100.0 C.
The force delivered perpendicularly to an object's surface per unit area across how that force is dispersed is known as pressure (symbol: p / P). The pressure in relation to the surrounding air pressure is known as gauge pressure, also spelt gauge pressure.
Pressure is expressed using a variety of units. Some of these are calculated by dividing a unit of force by a unit of area; for instance, the metric system's unit of pressure, a pascal (Pa), is equal to one newton / square metre (N/m2).
P₁/T₁=P₂/T₂
2815 ×373/423=2482.2torr
To know more about pressure, here:
https://brainly.com/question/12971272
#SPJ1