Answers
Answer:
the study of living organisms, divided into many specialized fields that cover their morphology, physiology, anatomy, behavior, origin, and distribution
Explanation:
the plants and animals of a particular area.
"the biology of Chesapeake Bay"
the physiology, behavior, and other qualities of a particular organism or class of organisms.
"human biology"
Related Questions
What is the source of the forces that move the tectonic plates along this plate
boundary?
A. Differences in density between two different types of oceanic
crust
OB. Convection currents in the mantle
C. Convection cur nts in the ocean
D. Differences in density between oceanic crust and continental crust
Answers
The source of the forces that move the tectonic plates along this plate
boundary is the convection current in the mantle. Hence, the correct
option in option (B)
What are Tectonic plates ?Tectonic plates refers to the Spheres that are found in the uppermost Mantle of the Earth .
The Tectonic plates are also called the Litosphere .
The Motion of the Plates about the axis causes the Tectonic demarcations which are Know as the
Convergent
Divergent
Transform
Now these Plate are formed by the Convection of Currents in the Earths's Mantle
That is, the Dynamics of Current in and around the Earths Mantle brings about the tectonic plates
Hence the the correct option in option (B)
Learn more about tectonic plates Here ;
brainly.com/question/15476626
#SPJ1
Convection currents in the mantle is the source of the forces that move the tectonic plates along this plate boundary and is denoted as option B.
What is Convection?This is referred to the process of heat transfer in a fluid such as gases and liquids.
The convection currents in the mantle is responsible for the force present in the tectonic plates.
Read more about Convection here https://brainly.com/question/9382711
#SPJ1
In three to five sentences, predict the bonding activity between Carbon and Chlorine. Explain why they would bond that way in terms of electronegativity and valence electrons.
Answers
Answer:
Carbon has 4 valence electrons and Chlorine has 7 valence electrons. The bonding between the atoms is due to electronegativity difference. The electronegativity of carbon is 2.5 compared to chlorine which is 3.1, so they will bond by sharing the electron pair (valance electron) from each atom in a covalent bond. In addition, Carbon is more electronegative than Chlorine, which means that it takes less energy to remove an electron from Carbon than from Chlorine. This makes it easier for the electrons to be shared between them (since there is less energy required to share an electron).
Explanation:
5. Write the two resonance hybrids for the carbocation that would be formed by protonation at C-1 of 2-methyl-1,3-pentadiene. Without doing a calculation, would you expect C-2 or C-4 (the two end carbons of the allylic cation) to have the most positive charge on it
Answers
Answer:
Follows are the solution to this question:
Explanation:
Please find the complete solution in the attached file.

A 4.36-g sample of an unknown alkali metal hydroxide is dissolved in 100.0 mL of water. An acid-base indicator is added, and the resulting sol utio n is titrated with 2.50 M HCl(aq) solution. The indicator changes color, signaling that the equivalence point has been reached, after 17.0 mL of the hydrochlor ic acid solution has been added. What is the molar mass of the metal hyd roxide?
Answers
We are given a sample of an unknown alkali metal hydroxide, and are being asked it's molar mass. If we were to consider this alkali metal hydroxide with HCl ( hydrochloric acid ), the following reaction would take place -
hydrochloric acid + sodium hydroxide → sodium chloride + water
Let me rewrite this reaction with respect to the " lettering " of the compounds,
HCl + MOH → MCl + H2O
M is replaced with the alkaline metal sodium in this case. From that, you can tell that this unknown alkali metal hydroxide is sodium hydroxide.
To solve for the number of moles of HCl, we can do the following -
n = C * V = 2.5 * 0.017 = 0.0425 moles,
n( MOH ) = n( HCl ) = 0.0425 moles
Using the formula n = m / M -
Molar Mass = m / n = 4.36 / 0.0425 ≈ 103 grams / mole
Solution = ( About ) 103 grams / moles of hydroxide
Which list only includes terms that describe water?
A) compound, element, pure
substance
B) compound, element, molecule
C) compound, mixture, molecule
D) compound, molecule, pure substance
Answers
Water is an inorganic substance made of oxygen and hydrogen atoms. Water can be said as a compound, molecule, and pure substance. Thus, option D is correct.
What is a pure substance?In chemistry, a pure substance is defined to be a substance that has distinct chemical characteristics and is known to have a constant and definite composition of matter all over.
A compound is a substance that is made of two or more elements joined chemically, while a molecule is the unit of the compound comprising of the atoms bonded by the forces.
Water is a pure substance as it has a definite composition, it is a compound and a molecule as it is made of two hydrogens and one oxygen atom that are linked through an attractive force.
Therefore, option D. a water molecule is a compound, molecule, and pure substance.
Learn more about pure substance here:
https://brainly.com/question/24462192
#SPJ2
Oxygen is a______________________ modulator of oxygen binding to hemoglobin.
Answers
Answer:
Oxygen is a positive modulator of oxygen binding to hemoglobin.
Explanation:
A positive modulator is one which on binding to an allosteric protein, enhances or increases the activity of that protein.
An allosteric protein is one in which when a ligand binds to one site affects the binding properties of another site on the same protein.
Hemoglobin is a protein that binds and transports oxygen in the body. It exists in two states, the T and R state. It has four binding sites for oxygen. When oxygen is not bound to the heme part of hemoglobin, it exists in the T state with little affinity for oxygen. However, when the first oxygen molecule binds to heme, it brings about conformational changes in the molecule converting it from the T state to the R, and hence it binds with more affinity for the the remaining three molecules of oxygen.
Therefore, oxygen is a positive modulator of oxygen binding to hemoglobin.
A buret dispenses 16.4 mL of a 0.521 M NaOH solution. How many moles of NaOH were dispensed?
Answers
The number of moles will be 8.54 mole.
The concentration can be expressed as the ratio of number of mole and volume . It can be shown as:
C= n/V
where, C is concentration, n is number of moles and V is volume.
The given data:
C = 0.521 M
V = 16.4 mL
n = ?
The number of moles can be determined by using the formula:
C = n/v
n = C×v....(i)
Put the values of given data in above equation.
n = 0.521 × 16.4
n = 8.54
Therefore, the number of moles will be 8.54 mole.
To know more about mole.
https://brainly.com/question/19860494
#SPJ1
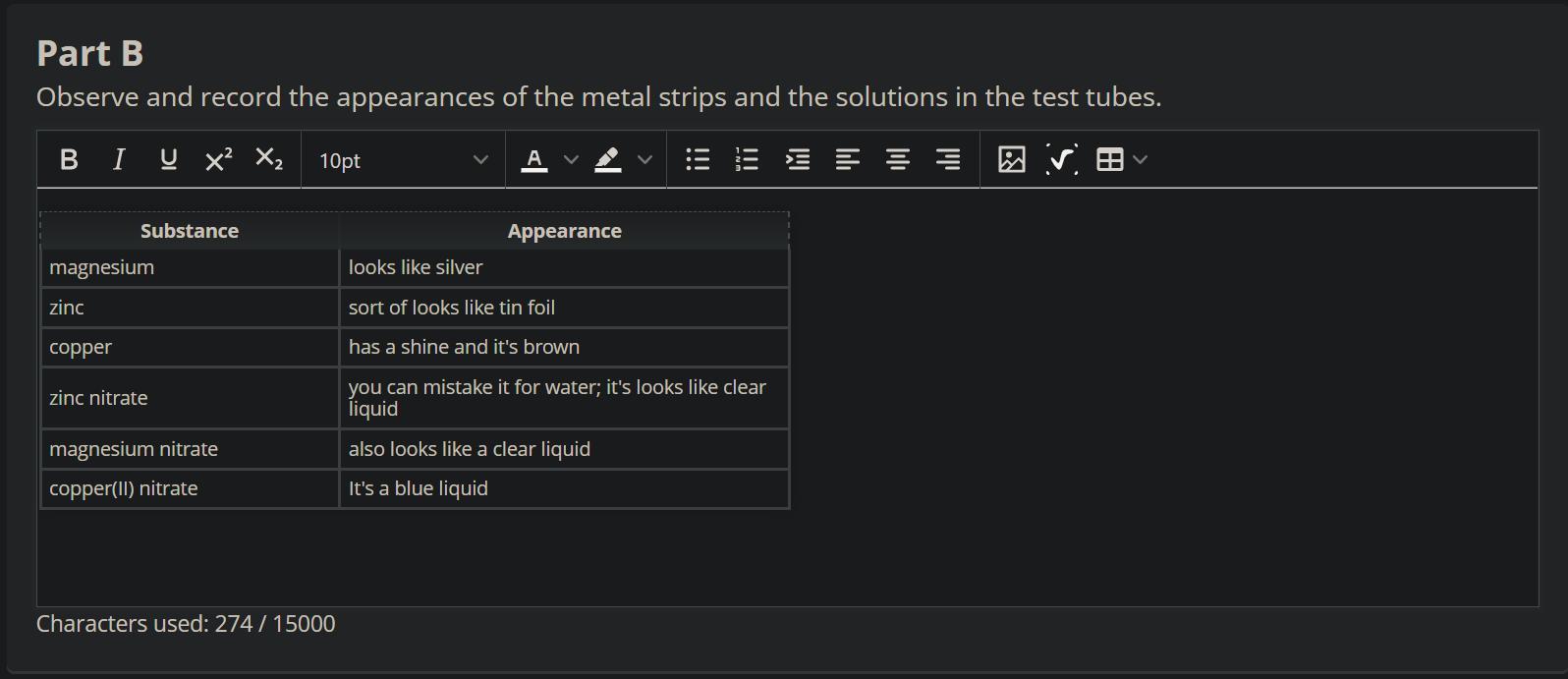
measure and record the masses of all metal strips you set out in front of the test tubes
( magnesium nitrate) ( zinc nitrate) ( copper nitrate) (magnesium ribbon) ( zinc strip ) (copper strip )
observe and record the appearances of the metal strips and the solutions in the test tubes

Answers
Magnesium ribbon dissolves in zinc nitrate and copper nitrate solutions.
Even though the question is incomplete and refers to your practical work, however, I will try to help you as much as I can.
Metals dissolves in solutions of other metals that are lower than them in the electrochemical series. Hence, copper strip will show no change in magnesium nitrate or zinc nitrate solution. A zinc strip will not show any change in magnesium nitrate or zinc nitrate.
However, a magnesium ribbon will dissolve very quickly in zinc nitrate and copper nitrate solutions.
Learn more: https://brainly.com/question/14396802
Look at the screenshot attached
Select the structure that corresponds
to the molecule name:
aniline
B.
A.
-NH₂
C. both
-NH₂
Enter

Answers
Answer:
B- \(C_{6} H_{5} NH_{2}\)Explanation:
Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine.


Calculate the mass of 6.023 x 10^4 molecules of manganese (IV) oxide(careful of your molecular formula).
Answers
1) First, we need to transform molecules into moles. For this, we use the avogadro constant:
6.023 × 10²³ moleculas ----- 1 mol
6.023 × 10⁴ molecules ------ x mol
x = 6.023 × 10⁴/ 6.023 × 10²³
x = 1 × 10⁻¹⁹ mol
2) Now we need to find the molecular formula of manganese (IV) oxide to find out its molar mass and transform 1 × 10⁻¹⁹ mol into grams (mass of it). The molecular formula of manganese (IV) oxide is MnO₂. So its molar mass: 54.94 + (2x16) = 86.94 g/mol
Now we use the following equation to find out the mass:
mass = moles × molar mass
mass = 1 × 10⁻¹⁹ × 86.94
mass = 86.94 × 10⁻¹⁹
mass = 8.7 × 10⁻¹⁸ grams
Answer: 8.7 × 10⁻¹⁸ grams
Geology
Please help!


Answers
The first image shows cleavage, same as the second. The third image shows fracture
What is shown in the images1. Image 1:
As it has clean surfaces and appears to have been sliced with a knife, it exhibits CLEAVAGE.Visible cleavage planes: 3There is not a 90-degree cleavage.Rhombohedral is the three-dimensional structure it symbolizes.2. Image 2
It exhibits CLEAVAGE.Visible cleavage planes: 1Since there is only one cleavage plane, cleavage angle is irrelevant.Sheet is the three-dimensional structure it symbolizes.Image 3:
It exhibits FRACTURE because it is fractured unevenly. In addition, the surfaces are not smooth.Inapplicable if there are obvious cleavage planesAngle of cleavage: inapplicableIt does not apply to the three-dimensional structure it portrays.Read more on rocks here:https://brainly.com/question/26046551
#SPJ1
Identify the fact that is FALSE about the law of conservation of energy?
Answers
Answer:
What are the options?
Explanation:
......
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Which of the following test tubes
(5 Points) would have the fastest rate of reaction? Defend your answer.
(5 Points) would have the slowest rate of reaction? Defend your answer.After presenting a reason and evidence in an argumentative essay, a writer should present
Answers
Answer:
could you explain your question more
Explanation:
Fission is the of a heavy nucleus into two smaller nuclei. This process is used in __________ .
Answers
Nuclear Fission
Nuclear fission, subdivision of a heavy atomic nucleus, such as that of uranium or plutonium, into two fragments of roughly equal mass. The process is accompanied by the release of a large amount of energy. In nuclear fission the nucleus of an atom breaks up into two lighter nuclei.
Nuclear fission is a process where the nucleus of an atom is split into two or more smaller nuclei, known as fission products. The fission of heavy elements is an exothermic reaction, and huge amounts of energy are released in the process.
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
Write balanced, net ionic equations for the following precipitation, acidn base, or gasn forming reactions. Include states of matter. Hint: You should write the molecular equation first to predict the products. a) mixing aqueous solutions of iron (III) chloride and lithium sulfide b) mixing aqueous solutions of sodium acetate and ammonium phosphate c) mixing aqueous solutions of perchloric acid and potassium hydroxide d) mixing aqueous solutions of ammonia and nitric acid e) mixing aqueous solutions of nitrous acid and sodium hydroxide f) adding aqueous hydroiodic acid to solid calcium carbonate g) Problems c) through f) are acid- base reactions. Comment on the differences in the net ionic equations for these reactions.
Answers
Answer:
Explanation:
a ) 2FeCl₃ + 3Li₂S = Fe₂S₃ ( s ) + 6 LiCl
2Fe⁺³ + 6Li ⁻ + 6Cl⁻ + 3S⁻² = 6Li + 6Cl⁻ + Fe₂S₃ ( s )
b )
3CH₃COONa +( NH₄)₃PO₄ = 3CH₃COONH₄ + Na₃PO₄
3CH₃COO + 3Na⁺ + 3NH₄⁻ + PO₄⁺³ = 3CH₃COO⁻ +3NH₄⁺ + Na₃PO₄
c )
HClO₄ + KOH = kClO₄ + H₂O
H ⁺ + ClO₄⁻ + K⁺ + OH⁻ = k⁺ ClO₄⁻ + H₂O
d )
NH₄OH + HNO₃ = NH₄NO₃ + H₂O
NH₄⁺ + OH⁻ + H⁺ + NO₃⁻ = NH₄⁺ + NO₃⁻ + H₂O
e )
HNO₂ + KOH = KNO₂ + H₂O
H⁺ + NO₂⁻ + K⁺ + OH⁻ = K⁺ + NO₂⁻ + H₂O
f ) HIO₃ + CaCO₃ ( s ) = Ca( IO₃ )₂ + H₂CO₃
H⁺ + IO₃⁻ + CaCO₃ ( s ) = Ca( IO₃ )₂ + H₂CO₃
g )
c ) is strong acid and strong base
d ) is weak base and strong acid
e ) weak acid and strong base
f ) Strong acid and basic salt
Predict the total pressure in Container C if the initial pressure in Container A was doubled and Container B was reduced by one-half, then mixed in Container C. Show your work.
Answers
The total pressure in Container C is \(5_{P}\)/(\(2_{V}\)). If the initial pressure in Container A was doubled and Container B was reduced by one-half.
To solve this problem, we need to use combined gas law, which relates with pressure, volume, and temperature of a gas;
(P₁V₁)/T₁ = (P₂V₂)/T₂
where P₁ and V₁ are initial pressure and volume, respectively, and T₁ is initial temperature. Similarly, P₂, V₂, and T₂ are inal pressure, volume, and temperature, respectively.
Let's assume that the volume and temperature are constant in all three containers. Therefore, we can simplify the equation to;
P₁/P₂ = V₁/V₂
We can use this equation to solve for the final pressure in Container C.
First, let's calculate the new pressures in Containers A and B;
Container A; the initial pressure was doubled, so P₁ = \(2_{P}\) and V₁ = V (since the volume is constant). Therefore, P₂ = P₁/(V₁/V₂) = \(2_{P}\)/(1/2) = \(4_{P}\).
Container B; the initial pressure was reduced by one-half, so P₁ = P/2 and V₁ = V (since the volume is constant). Therefore, P₂ = P₁/(V₁/V₂) = (P/2)/(1/2) = P.
Now that we have the new pressures in Containers A and B, we can use them to find the total pressure in Container C:
Container C; we are mixing equal volumes of gases from Containers A and B, so the total volume is \(2_{V}\). The total pressure is the sum of the partial pressures of the gases in Containers A and B, which are \(4_{P}\) and P, respectively. Therefore, the total pressure in Container C is:
\(P_{total}\) = (\(4_{P}\) + P)/(\(2_{V}\))
= \(5_{P}\)/(\(2_{V}\))
So, the final pressure in Container C is \(5_{P}\)/(\(2_{V}\)).
To know more about combined gas law here
https://brainly.com/question/13154969
#SPJ1
You come across the following container while working in the lab: Answer the following questions in the space below: 1. Identify the WHMIS symbols. 2. What precautions should you take and why?
Answers
Type #1 Flame symbols are among the WHMIS emblems.
Type 2: Symbols with a flame above a circle.
Exploding bomb symbols are of type 3.
Compressed gas symbols are of type 4.
Corrosion symbols are type #5.
Skull and water the water symbols are type #6.
Exclamation mark symbols are type #7.
Health hazard symbols are type #8.
Because workplaces require a defined technique to detect hazardous items, WHMIS labels are crucial.
What does the WHMIS stand for?The national ’s hazard standard for Canada is the Health And Safety At work System (WHMIS). Hazard categorization, cautionary container labeling, the distribution of safety data sheets, and worker information and training programs are the system's main components.
What does WHMIS look like in the US?The U.S. Ohs Hazard Identification Standard and WHMIS are quite similar.
To know more about WHMIS visit:
https://brainly.com/question/28542158
#SPJ1
Place these hydrocarbons in order of decreasing boiling point. Rank from highest to lowest boiling point. 1. Paraffin, C40Hg2 2. Propane, G2H53. Hexane, C6H14 4. 2,2-dimethylbutane, C6 H14 5. Heptadecane, C17H36A. Highest BPB. Highest BP
Answers
Answer:
Paraffin > Heptadecane > Hexane > 2,2-dimethylbutane > Propane
Explanation:
It must first be establish that all the molecules listed in the question are alkanes. For alkanes, the intermolecular forces between the molecules of alkanes increases with increasing molecular weight. This is as a result of increase in the surface area of the molecule. Increase in surface area implies a greater degree of dispersion forces.
This is the reason why high boiling points are observed for high molecular weight alkanes.
Explain in as much detail as you can why hydrochloric acid, which is a strong acid, and ethanoic acid, which is a weak acid, can both have a pH of 5.
Answers
Answer:
No
Explanation:
Strong acids are from the pH level 1-3while weak acids are from the pH level 4-6
A student dissolves of glucose in of a solvent with a density of . The student notices that the volume of the solvent does not change when the glucose dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to significant digits. molarity molality
Answers
Answer:
0.052 M
0.059 m
Explanation:
There is some missing info. I think this is the complete question.
A student dissolves 4.6 g of glucose in 500 mL of a solvent with a density of 0.87 g/mL. The student notices that the volume of the solvent does not change when the glucose dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.
Step 1: Calculate the moles of glucose (solute)
The molar mass of glucose is 180.16 g/mol.
4.6 g × 1 mol/180.16 g = 0.026 mol
Step 2: Calculate the molarity of the solution
0.026 moles of glucose are dissolved in 500 mL (0.500 L) of solution. We will use the definition of molarity.
M = moles of solute / liters of solution
M = 0.026 mol / 0.500 L = 0.052 M
Step 3: Calculate the mass corresponding to 500 mL of the solvent
The solvent has a density of 0.87 g/mL.
500 mL × 0.87 g/mL = 435 g = 0.44 kg
Step 4: Calculate the molality of the solution
We will use the definition of molality.
m = moles of solute / kilograms of solvent
m = 0.026 mol / 0.44 kg = 0.059 m
Consider the reaction
2NO(g) + O2(g) = 2NO2(g)
Suppose that at a particular moment during the reaction nitric oxide
(NO) is reacting at the rate of 0.066 M/s. (a) At what rate is NO2
being formed? (b) At what rate is molecular oxygen reacting?
Answers
Answer:
(a) Rate of formation of NO2 is also 0.066M/s
(b) Rate of reaction of O2 gas is 0.033M/s
Explanation:
(a) in one second, according to the equation,
2 moles of NO combines with 2moles of NO2.
Therefore 0.066M NO will still consume 0.066mole NO2.
(b) According to the equation,
2 moles NO consumes 1 mole O2, 0.0666M will consume 0.0333 mole O2
Please answer this!
What are the half-reactions for electrolytic cell with aluminum and gold
electrodes?
A. Al³+ (aq) + 3e → Al(s) and Au(s) → Au* (aq) + e
B. Al³+ (aq) + 3e → Al(s) and Aut(aq) + e¯ → Au(s)
► Au(s).
C. Al(s) → A1³+ (aq) + 3e and Au* (aq) + e →
D. Al(s) → Al³+ (aq) + 3e¯ and Au(s) → Au*(aq) + e

Answers
Which physical property is best used to separate the two types of substances
in the mixture?
A. Particle size
B. Particle density
C. Magnetic attraction
D. Boiling point
Answers
Answer:
Magnetic attraction
Explanation:
Physical characteristics would be used to distinguish the components of a mixture, including such scale, form, colour, weight, magnetic fields or the potential to drop or floating. As material is moved into a filter and the solid wastes are filtered out.
Vanadium forms a complex with F- this complex. that has a charge of -3, and in which the oxidation state of the vanadium atom is +3. Name one possible geometry for
Answers
The comples has 6 ligands the geometry is Octahedral.
What is Octahedral?Octahedral is a geometric shape that has eight faces, each of which is an equilateral triangle. The shape has six vertices, and each vertex is connected to four other vertices, creating a three-dimensional structure. Octahedral shapes are commonly found in nature, but they can also be found in architecture and art. They are used as decoration on buildings and as part of sculptures. Octahedral shapes are often used to represent the elements in chemistry and can also be used to represent atoms and molecules. They are also used in mathematics, particularly in group theory, to represent the symmetries of an object.
The One of the Geometry can be Octahedral.
F-1: negative ligand
Central metal vanadium (V), with +3 oxidation state and complex have -3 change.
Based on this information we are going to find number of ligands (F-1)
[VFx]-3
Where,
-3 is the complex charge
V oxidation state is +3
x is the number of ligands.
So,
(+3) + x (-1) = -3
3 – x = 3
x = 6
This means this complex have 6 ligands.
Hence the complex is,
[VF6]-3
And if the comples has 6 ligands the geometry is Octahedral.
To learn more about the Oxidation State:
https://brainly.com/question/25551544
#SPJ1
(6
32. Calcium carbide, CaC2, is an important preliminary chemical for industries producing
synthetic fabrics and plastics. CaC2 may be produced by heating calcium oxide with
CaO + C CaC2+CO
What is the mass of CaC2 which can be produced from the reaction of excess calcium oxi
10.2 g of carbon? (Hint: Balance the equation first)
Answers
Answer:
18.1 g
Explanation:
Step 1: Write the balanced equation
CaO + 3 C ⇒ CaC₂ + CO
Step 2: Calculate the moles corresponding to 10.2 g of C
The molar mass of C is 12.01 g/mol.
10.2 g × 1 mol/12.01 g = 0.849 mol
Step 3: Calculate the moles of CaC₂ produced from 0.849 moles of C
The molar ratio of C to CaC₂ is 3:1. The moles of CaC₂ produced are 1/3 × 0.849 mol = 0.283 mol
Step 4: Calculate the mass corresponding to 0.283 moles of CaC₂
The molar mass of CaC₂ is 64.10 g/mol.
0.283 mol × 64.10 g/mol = 18.1 g
What concentration of Br−
results when 777 mL
of 0.733 M KBr
is mixed with 693 mL
of 0.461 M FeBr2?
Answers
Formula:
C1V1 + C2V2 = C3V3
Where C1 and V1 are the concentration and volume of the first solution, C2 and V2 are the concentration and volume of the second solution, and C3 and V3 are the concentration and volume of the resulting mixture.
Substituting the given values into the formula, we get:
(0.733 M)(0.777 L) + (0.461 M)(0.693 L) = C3(0.777 L + 0.693 L)
Simplifying the equation:
0.57084 + 0.31923 = 1.47 C3
0.89007 = 1.47 C3
C3 = 0.606 M
Therefore, the concentration of Br- in the resulting mixture is 0.606 M.
what is the 3rd element on the periodic table?
Answers
Answer:
Lithium
Explanation:
The 3rd (third) element on the periodic table is Lithium. Lithium is a soft, silvery, light alkali metal denoted with the symbol Li.
Hurry hurry plsplslslslslsl?!?!?!?!

Answers
Answer:
The rest of the food chain will not have as much to eat and will decrease in population and the decomposers will grow in population because the rest of the creatures will die and give the decomposers more food
Explanation:
Answer:
it would increase.
Explanation:
if light is not provided to the producer,the animals rate of death would certainly increase,making the decomposing level faster than normal