Answers
The mass of barium sulfate that can be formed from 196.0 grams of sulfuric acid is 466.78 g, rounded to three decimal places.
What is the mass?
To solve this problem, we need to use the balanced chemical equation for the reaction between sulfuric acid (H₂SO₄) and barium chloride (BaCl₂) to form barium sulfate (BaSO₄) and hydrochloric acid (HCl):
H₂SO₄ + BaCl₂ → BaSO₄ + 2HCl
According to the balanced equation, one mole of sulfuric acid reacts with one mole of barium sulfate to form one mole of barium sulfate.
The molar mass of sulfuric acid (H₂SO₄) is 98.079 g/mol, while the molar mass of barium sulfate (BaSO₄) is 233.39 g/mol.
Using the given mass of sulfuric acid, we can calculate the number of moles of sulfuric acid present as:
196.0 g / 98.079 g/mol = 2.000 moles
Since the reaction is 1:1 between sulfuric acid and barium sulfate, we know that 2.000 moles of barium sulfate can be formed.
To calculate the mass of barium sulfate formed, we can use the molar mass of barium sulfate:
mass of BaSO₄ = 2.000 moles * 233.39 g/mol = 466.78 g
Therefore, the mass of barium sulfate that can be formed from 196.0 grams of sulfuric acid is 466.78 g, rounded to three decimal places.
To know more anout mass, visit:
https://brainly.com/question/19694949
#SPJ1
Complete question is: The mass of barium sulfate that can be formed from 196.0 grams of sulfuric acid is 466.78 g, rounded to three decimal places.
Related Questions
how does a decrease in potential energy during a chemical rwaction relate to the temperature of the surroundings
Answers
Answer: If the potential energy of the reaction system decreases, then kinetic energy in the surroundings increases and the temperature of the surroundings rises
Explanation:
The decrease in the potential energy results in an increase in the temperature of the system and surroundings.
Mechanical energy has been described as the sum of the kinetic and the potential energy in the system. With the increase in the potential energy, there has been a decrease in the kinetic energy and vice versa.
The decrease in the potential energy results in an increase in the kinetic energy of the system. The kinetic energy has been termed as the energy of performing work. The rise in the kinetic energy results in an increase in the work done in the system and there has been an increase in the temperature of the system.
Thus, the decrease in the potential energy results in an increase in the temperature of the system and surroundings.
For more information about the potential energy, refer to the link:
https://brainly.com/question/21288807
The models show the phases of a substance at various temperatures. Which model BEST communicates the kinetic energy and particle arrangement of a liquid? A. Model D, because it has high kinetic energy so the particles in the model have ionized B. Model B, because it has some kinetic energy and the particles have a free flowing shape C. Model C, because it has high kinetic energy so the particles in the model have no definite shape D. Model A, because it has some kinetic energy and the particles move freely
Answers
Answer: Model B. I have included a graph for this question. Model D is incorrect because that is a model for plasma as liquid doesn't contain ionized particles. Model C is incorrect as that represents a solid. Model A is incorrect because that is showing a gas; the particles are moving very freely

According to the forces of attraction, model B communicates the kinetic energy and particle arrangement of a liquid.
What are forces of attraction?Forces of attraction is a force by which atoms in a molecule combine. it is basically an attractive force in nature. It can act between an ion and an atom as well.It varies for different states of matter that is solids, liquids and gases.
The forces of attraction are maximum in solids as the molecules present in solid are tightly held while it is minimum in gases as the molecules are far apart . The forces of attraction in liquids is intermediate of solids and gases.
The physical properties such as melting point, boiling point, density are all dependent on forces of attraction which exists in the substances.
Learn more about forces of attraction,here:
https://brainly.com/question/23841038
#SPJ2
Your question is incomplete but most probably your full question was, The models show the phases of a substance at various temperatures. Which model BEST communicates the kinetic energy and particle arrangement of a liquid? A. Model D, because it has high kinetic energy so the particles in the model have ionized B. Model B, because it has some kinetic energy and the particles have a free flowing shape C. Model C, because it has high kinetic energy so the particles in the model have no definite shape D. Model A, because it has some kinetic energy and the particles move freely

Things that you learn through your senses during an experiment are
A. hypotheses
B. controls
C. observations
D. conclusion
Answers
Answer:
c- observations
hope this helps
Explanation:
HELP PLEASE!!!!!
Can someone please help me I should submit it today and the answer should be as paragraph

Answers
Answer:
National fire protection Association
Explanation:
the nfpa is a global self funded nonprofit orgnazation establised in 1896 devoted to eliminating death injury protery loss and ecomomic loss due to fire and electrical hazards
Write the equation for the equilibrium constant (K) of the reaction studied in this exercise.
2C04 2- (ag) + 2Ht (ag) = CI20, 2- (ag) + H20(1)
Answers
The equation for the equilibrium constant (K) of the reaction studied in this exercise can be written as follows: K = ([\(CI_20\), 2-] * [\(H_20\)(1)]) / ([\(C0_4^ 2\)-] * [Ht])
In this equation, the concentrations of the species involved in the reaction are represented by the square brackets [ ]. The subscripts indicate the stoichiometric coefficients of each species in the balanced chemical equation.
The reaction being studied involves the following species:
\(C0_4^ 2\)- (ag) + 2Ht (ag) = \(CI_20\), 2- (ag) + \(H_20\)(1)
In the equilibrium constant expression, the concentration of \(CI_20\), 2- is multiplied by the concentration of \(H_20\)(1) and divided by the product of the concentrations of \(C0_4^ 2\)- and Ht. The stoichiometric coefficients in the balanced equation are used as exponents for the concentrations of the respective species.
It is important to note that the concentrations used in the equilibrium constant expression should be in molar units (mol/L) or expressed as partial pressures for gases.
Additionally, the equilibrium constant is specific to a given temperature, and its value provides information about the relative amounts of reactants and products at equilibrium.
For more such question on equilibrium constant visit:
https://brainly.com/question/3159758
#SPJ8
The weak base ionization
constant (Kb) for C4H4N2 is
equal to

Answers
Answer:
A
Explanation:
The weak base ionization constant (Kb) for \(C_{4} H_{4} N_{2}\) is equal to Option A.
Are weak foundations made of ionized?A weak foundation is a base that makes ionize only slightly in an aqueous solution. Remember that a foundation can be defined as an object, which receives a hydrogen ion from another object.
What is the kb of a weak foundation?To obtain an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (Ka). Similarly, the constant equilibrium of a weak base reaction with water is the base ionization constant (Kb). In any conjugate acid-base pair, KaKb = Kw.
Learn more about ionization here: brainly.com/question/25676623
#SPJ2
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
2.
Which form of cell division produces four cells with chromosomes different from the
parent cell?
B
budding
dominant
meiosis
Dmitosis
Answers
Answer:
meiosis
Explanation:
Completely describe the electrolytic cell corresponding to the following equation. (Hint: you may need to combine 2 half reactions from Table 17-1 to make one of the half reactions for this cell)
Cr2O7^2– + I^– → Cr^3+ + IO3^–
With work please
Answers
The first half-reaction is the oxidation of Cr2O7^2– to Cr^3+ and the second half-reaction is the reduction of I^– to IO3^–. When combined, the overall reaction is Cr2O7^2– + I^– → Cr^3+ + IO3^–.
The electrolytic cell consists of two electrodes, one anode and one cathode, both of which are immersed in an electrolyte solution. At the anode, the Cr2O7^2– ions are oxidized to Cr^3+ ions, releasing electrons into the external circuit.
At the cathode, the I^– ions are reduced to IO3^– ions, and the electrons from the external circuit are used to drive the reaction. The electrolyte solution must contain both Cr2O7^2– and I^– ions in order to facilitate the transfer of electrons between the electrodes.
The overall reaction is driven by the potential difference between the anode and the cathode, which is created by the flow of electrons through the external circuit.
Learn more about electrolytic cell at:
https://brainly.com/question/4030224
#SPJ1
PLEASE HELP BRAINLIEST AND 15 points.
1. Which substance is nonvolatile ?
(1.5 Points)
Substance B, boiling point of 105 °C
Substance C, boiling point of 25 °C
Substance A, boiling point of 75 °C
Substance d, boiling point of 45 °C
Answers
Answer:
Substance B, boiling point of 105 °C
Explanation:
Non volatile substances have high boiling points
Which shape is being shown by the model?

Answers
6. Who stated that matter is not composed of particles
Answers
After careful consideration your answer is...
Leucippus and Democritus
*Hope I helped*
~Alanna~
Answer:
The first theories of matter were put forward by Empedocles in 450 BC, he proposed that all matter was composed of four elements - Earth, air, fire and water. Later, Leucippus and Democritus suggested matter was made up of tiny indestructible particles continuously moving in empty space.
Explanation:
make a 1:10 dilution of serum, redilute 1:10, and redilute once more 1:2. what is the final dilution? (show how you solved this problem)
Answers
the final dilution is 1:200. A 1:10 dilution of serum means that 1 part serum is mixed with 9 parts diluent (usually water or saline).
To make this dilution, we can take 1 mL of serum and mix it with 9 mL of diluent.
To redilute 1:10, we take 1 mL of the 1:10 diluted serum and mix it with 9 mL of diluent. This results in a 1:100 dilution, since we have diluted the serum by a factor of 10 again (1:10 x 1:10 = 1:100).
To redilute 1:2, we take 1 mL of the 1:100 diluted serum and mix it with 1 mL of diluent. This results in a 1:200 dilution, since we have diluted the serum by a factor of 2.
To calculate the final dilution, we need to multiply the dilution factors together:
1:10 x 1:10 x 1:2 = 1:(10 x 10 x 2) = 1:200
Therefore, the final dilution is 1:200.
To know more about dilution, visit: brainly.com/question/28997625
#SPJ4
what is fart made out of
Answers
с
app.101edu.co
Question 2 of 18
Liquid butane is used in cigarette lighters. The boiling point of butane
at 1 atm pressure is -1.0 °C and its AH(vap) is 22.44 kJ/mol.
R = 8.314 x10-3 kJ/mol K.
Calculate the pressure (in atm) of the butane in the lighter at 25.0 °C.
Answers
The vapor pressure of butane in the lighter at 25.0 °C is approximately 0.493 atm.
How to calculate the pressureWe are given that the boiling point of butane at 1 atm pressure is -1.0 °C, which is equivalent to 272.15 K. At this temperature, the vapor pressure of butane is 1 atm. We are also given that the enthalpy of vaporization of butane is 22.44 kJ/mol and R = 8.314 x 10⁺³ kJ/mol K.
We want to find the vapor pressure of butane at 25.0 °C, which is equivalent to 298.15 K. Substituting these values into the Clausius-Clapeyron equation, we get:
ln(P2/1 atm) = (-22.44 kJ/mol / (8.314 x 10⁻³ kJ/mol K)) * (1/298.15 K - 1/272.15 K)
Solving for P2, we get:
P2 = 1 atm * e⁻²² kJ/mol / (8.314 x 10⁻³ kJ/mol K)) * (1/298.15 K - 1/272.15 K)]
P2 = 0.493 atm
Learn more about pressure on
https://brainly.com/question/28012687
#SPJ1
Why was it necessary to make sure that some solid was present in the main solution before taking the samples to measure Ksp?
Answers
The question is incomplete, the complete question is;
Why was it necessary to make sure that some solid was present in the main solution before taking the samples to measure Ksp? Select the option that best explains why. Choose... A. To make sure no more sodium borate would dissolve in solution. B. To ensure the dissolution process was at equilibrium. C. To make sure the solution was saturated with sodium and borate ions. D. All of the above
Answer:
B. To ensure the dissolution process was at equilibrium.
Explanation:
The solubility product is a term used in chemistry to describe the equilibrium between the dissolved, dissociated and undissolved solute of a relatively low solubility ionic solid.
For an ionic solid MX, the solubility product is given as ;
MX(s) ----> M^n+(aq) + X^n-(aq)
If Ksp indeed scribes an equilibrium process for dissolution, it then implies that some undissolved solute must be present before samples are taken to measure the Ksp of a sample. This ensures equilibrium between dissolved and undissolved solute.
B. To provide the dissolution process was at equilibrium and assure equilibrium between dissolved and then undissolved solute.
What is a Solubility Product?
The solubility product is a representation accustomed in chemistry to describe the equilibrium between the dissolved, dissociated, and as they are undissolved solutes of a relatively low solubility ionic solid.
For an ionic solid MX, the solubility product is given as;
Then, MX(s) ----> M^n+(aq) + X^n-(aq)
If Ksp scribes an equilibrium process for dissolution, it then means that some undissolved solute must be attending before samples are taken to measure the Ksp of a sample.
This confirms equilibrium between dissolved and also undissolved solute between dissolved and undissolved solute.
Find more innformation about Solubility Product here:
https://brainly.com/question/13155939
The question is incomplete, the complete question is;
Why was it necessary to make sure that some solid was present in the main solution before taking the samples to measure Ksp? Select the option that best explains why. Choose... A. To make sure no more sodium borate would dissolve in solution. B. To ensure the dissolution process was at equilibrium. C. To make sure the solution was saturated with sodium and borate ions. D. All of the above
Prepare one solution that has 0.12 M of FeCl3 and 0.40 M of HCl with the reagents 3 M HCl and Solid FeCL3 * 6H20. Provide the calculations and protocol to make the solution in a lab.
Answers
To prepare a 0.12 M solution of FeCl₃, the amount of solid FeCl₃ to be dissolved in a given volume of solvent will be 9.72 grams.
Given,
Molarity of FeCl₃ (M)= 0.12 M
The molecular weight (m) of FeCl₃ is = 162 gm
The volume of the solution (V) to be prepared is =500 ml
The amount of FeCl₃ to be dissolved to make a 0.12 M solution is= x
So,
MV= x ÷ m × 1000
0.12× 500 = x ÷ 162 × 1000
x = 60 × 162 ÷ 1000
x= 9.72 gm
So 9.72 grams of FeCl₃ is dissolved to make 500 ml of 0.12 M solution.
For preparing 0.4 M HCl from 4M HCL:
If we need to make 500 ml of solution with 0.4M of HCL, then we use the formula:
M₁V₁= M₂V₂
0.4 × 500= 4 × x
x= 50 ml
So 50 ml of 4M HCL is taken to make 0.4 M HCL.
To learn more about FeCl₃, refer to the link:
https://brainly.com/question/32098087
#SPJ1
Half-life
Nickel-63 has a half life of 92 hours. If a 1000 gram
sample decayed for 368 years
, how much
Nickel -63 remains?
Answers
What type of reaction is C3H6O + O2 --> CO2 + 3 H2O
Answers
Answer:
Combustion of propanone I believe
Explanation:
A Se ion has a mass number of 77 and a charge of −2
. Determine the number of neutrons, protons, and electrons in this ion.
number of neutrons:
number of protons:
number of electrons:
Answers
Answer:
neutrons = 71
protons = 6
electrons = 6
Explanation:
Hope this works :)
An atom has the atomic numbers 6 and 8 neutrons, what is the mass number?
Answers
Answer: Mass number will be 14.
Explanation:
Given : 6 and 8 protons and neutrons.
Solution :
According to the formula of Atomic mass number,
Atomic mass number = protons+ neutrons
= 6 + 8
= 14
If 500.0 kJ of energy is released in the synthesis of water from its elements, how many grams of water are formed?
O2(g) + 2H2(g) → 2H₂O(l) + 572 kJ
Answers
15.6 grams of water are formed when 500.0 kJ of energy is released in the synthesis of water from its elements.
Grams of water calculation.
The balanced chemical equation for the synthesis of water from its elements is:
O2(g) + 2H2(g) → 2H₂O(l) + 572 kJ
The given energy change is -500.0 kJ, which means that energy is released during the reaction.
We can use the relationship between the amount of energy released and the amount of substance involved in the reaction to calculate the mass of water formed.
The molar enthalpy change for the reaction can be calculated as follows:
ΔH = -500.0 kJ/mol H2O
The molar enthalpy change tells us how much energy is released when 1 mole of water is formed.
The molar mass of water (H2O) is 18.015 g/mol.
To calculate the mass of water formed, we can use the following equation:
mass of water = (ΔH / molar enthalpy change) x molar mass of water
mass of water = (-500.0 kJ / mol H2O / -572 kJ/mol) x 18.015 g/mol
mass of water = 15.6 g
Therefore, 15.6 grams of water are formed when 500.0 kJ of energy is released in the synthesis of water from its elements.
Learn more about grams of water below.
https://brainly.com/question/24258132
#SPJ1
You have 13.2 mols of CO2 . Calculate the mass of CO2.
Answers
• Given mol of CO2 = 13.2 mols
Molar Mass of CO2 = ( 12+(16*2) = 44g/mol
Mass can be calculated by the following :
• Moles of CO2 = m (weight) / Molar Mass
weight = Molar Mass * moles of CO2
= 44 * 13.2 = 580.8 grams
Therefore weight (mass) of CO2 = 580.8 g
Which of the following statements about subatomic particles are correct? (select all that apply)
A. Neutrons and protons are the only charges subatomic particles
B. The charge on a proton is equal in magnitude and opposite in sign to that of an electron
C. The mass of a proton is similar to that of a neutron
D. The mass of a neutron is slightly greater than that of an electron
Answers
The following statements about subatomic particles are correct:
B. The charge on a proton is equal in magnitude and opposite in sign to that of an electron
C. The mass of a proton is similar to that of a neutron
D. The mass of a neutron is slightly greater than that of an electron.
Subatomic particles refer to the basic building blocks of matter that make up atoms. The main subatomic particles are:
Protons: Positively charge particles found in the nucleus of an atom. The number of protons in an atom determines the atomic number, and thus the identity, of the element.
Neutrons: Neutral particles found in the nucleus of an atom. The number of neutrons in an atom can vary, resulting in isotopes of the same element.
Electrons: Negatively charged particles that orbit the nucleus of an atom. The number of electrons in an atom determines its chemical properties and reactivity.
These subatomic particles interact with each other through electromagnetic forces to form the atoms and molecules that make up matter. Understanding the behavior and interactions of subatomic particles is key to understanding the properties and behavior of matter on a macroscopic scale.
Learn more about charge here:
https://brainly.com/question/19886264
#SPJ4
what are atoms and molecules and elements In a essay
Answers
Atoms are the smallest indivisible part of an element while molecules are combinations of two or more atoms. See details below.
What are atoms and molecules?Atoms are the smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.
Molecules, on the other hand, are the smallest particle of a specific element or compound that retains the chemical properties of that element or compound. They are two or more atoms held together by chemical bonds.
An element is the simplest chemical substance that cannot be decomposed in a chemical reaction or by any chemical means and made up of atoms all having the same number of protons.
Learn more about atoms and molecules at: https://brainly.com/question/7013021
#SPJ1
Select the statements that are true regarding open tubular and packed columns in gas chromatography.
A. For similar analysis times, open tubular columns provide better resolution than packed columns.
B. Packed columns offer increased sensitivity to small sample sizes.
C. Packed columns have a greater sample capacity than open tubular columns.
D. Open tubular columns give better resolution but longer analysis separation times compared to packed columns.
E. Packed columns give broader peaks with longer retention times.
Answers
Answer:
true or false :-
a) yes
b) no
c)yes
d)yes
e)no
For similar analysis times, open tubular columns provide better resolution than packed columns, Packed columns have a greater sample capacity than open tubular columns and Open tubular columns give better resolution but longer analysis separation times compared to packed columns are true regarding open tubular. Hence, options A, C and D are correct.
What is gas chromatography used for?The chemical constituents of a sample mixture are identified, separated, and quantified using an analytical technique called gas chromatography.
Gas chromatography is a technique for separating compounds in mixtures by injecting a gaseous or liquid sample into a mobile phase, which is usually referred to as the carrier gas, and passing the gas through a stationary phase. The mobile phase is often composed of an inert gas or an unreactive gas, such as helium, argon, nitrogen, or hydrogen.
Thus, options A, C and D are correct.
For more information about gas chromatograph, click here:
https://brainly.com/question/13565117
#SPJ2
Why does boron not react directly with hydrogen
Answers
Answer:
that's a good question but I'm not too sure
Which of these does not accurated descibe the wright brothers first airplane
Answers
Answer:
it had wings that flapped similat to birds wings
a wave has a frequency of 240 hz and a wavelength of 3.0 m. what is the speed of the wave
Answers
Answer:
The speed of the wave is
v = ( 240 ) ( 3.0 )= 720.0 m/s
Explanation:
If n is the frequency of the wave λ is the wavelength of the wave. v is the speed of the wave,
the three quantities are related by v = n λ
We have, n = 240 Hz λ = 3.0 m v =?
I Hope This Makes Sense
What is the length of the paper clip in cm
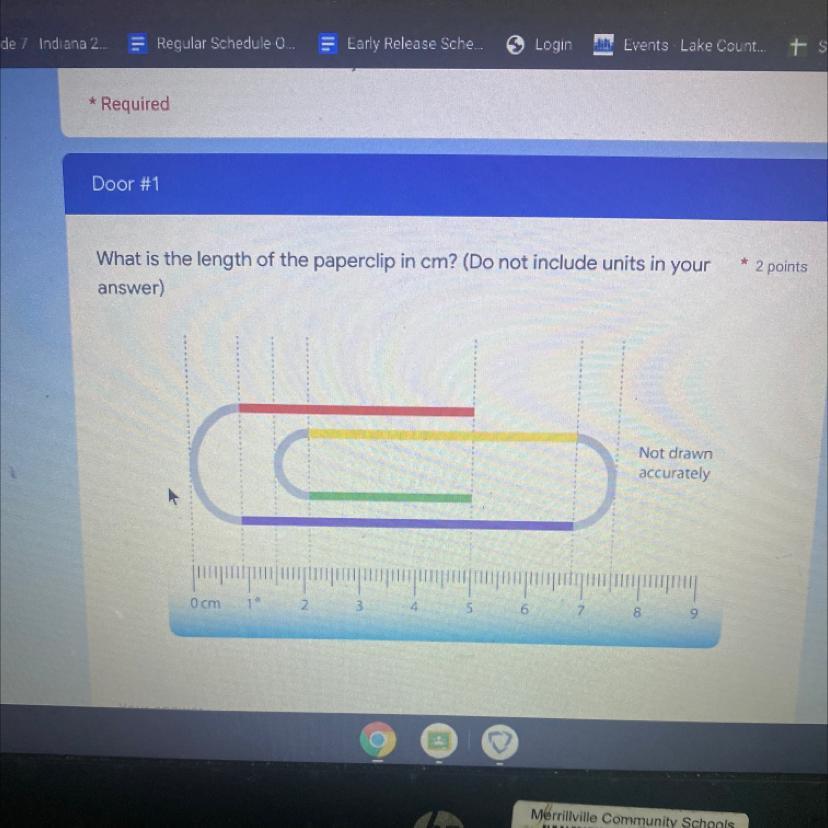
Answers
Answer:
24.5 cm
Explanation:
It would be really complicated to type out, so I've attached an image of how I solved this:
*I separated the paperclip into different sections, figured out the length of those sections, and added them together.
(sorry that my work isn't the neatest)
