WHAT MAKES ELECTRONS JUMP TO OTHER ORBITS AROUND THE NUCLEUS THEN FALL AGAIN
Answers
Answer:
Explanation:
Electrons absorb energy in photons. This makes the eclectron excited. An electron in an excited state can release energy and fall to a lower orbit
Related Questions
C6H5COOH(s) -- C6H5COO-(aq) + H+(aq)
Ka = 6.46 x 10e-5
Benzoic acid, C6H5COOH, dissociates in water as shown in the equation above. A 25.0 mL sample of an aqueous solution of pure benzoic acid is titrated using standardized 0.150 M NaOH.
After addition of 15.0 mL of the 0.150 M NaOH, the pH of the resulting solution is 4.37. Calculate the following:
The number of moles of NaOH added.
Please show steps.
Thank you in advance!
Answers
The number of moles of NaOH added is 0.00225 mol.
To calculate the number of moles of NaOH added, we can use the stoichiometry of the reaction between benzoic acid (C6H5COOH) and NaOH. According to the balanced equation, 1 mole of benzoic acid reacts with 1 mole of NaOH. Given that the concentration of NaOH is 0.150 M and 15.0 mL of NaOH solution is added, we can first convert the volume to liters by dividing it by 1000:
Volume of NaOH = 15.0 mL / 1000 mL/L = 0.015 L
Next, we can calculate the number of moles of NaOH using the formula:
moles of NaOH = concentration × volume
moles of NaOH = 0.150 M × 0.015 L = 0.00225 mol
Therefore, the number of moles of NaOH added is 0.00225 mol.
To know more about C6H5COOH, click here https://brainly.com/question/29206874
#SPJ11
An organic compound of molecular formula c3h6o there are 2 compound having ame formula A ha fruity mell while B releae hydrogen with mg identify a and b and give chem reaction for the proce involved and ugget a method to convert b to a and what relation between A and B why
Answers
Structural and Organic Formula of Acetone: Acetone Formula (C3H6O) (Propanone) So, Both propanal and propanone are isomers of the substance having the chemical formula C 3 H 6 O.
What is the straightforward meaning of compound?A substance in science that is created through the chemical joining of two or more distinct elements Table salt (NaCl), which is derived from the elements sodium and chloride, and water (H2O), which is created from the elements hydrogen and oxygen, are two examples of compounds.
Why is water a compound?Because water molecules make up its composition, water is a compound. Atoms made of water don't exist. Hydrogen and oxygen atoms are found in a certain ratio of two hydrogen atoms to one oxygen in the structure of water molecules.
To know more about Compound visit:
https://brainly.com/question/19458442
#SPJ4
what one test (not using the semicarbazoneor 2,4-dnp derivatives) could be used to differentiate between thefollowing pairs? describe what would you observe? a) 2-pentanone and 3-pentanone b) pentanol and 3-pentanone c) 1-propanol and 2-propano
Answers
To differentiate between the given pairs of compounds, we can use the iodoform test. This test involves treating the compounds with iodine and sodium hydroxide solution.
The observation of a yellow precipitate indicates the presence of a methyl ketone (2-pentanone), while the absence of a precipitate suggests the presence of an ethyl ketone (3-pentanone). In the case of pentanol and 3-pentanone, the iodoform test cannot differentiate between them as both compounds lack a methyl ketone group.
Lastly, in the differentiation between 1-propanol and 2-propanol, the iodoform test is not applicable since both compounds lack a methyl ketone group.
2-Pentanone and 3-Pentanone differentiation:
Perform the iodoform test by adding iodine solution to a test tube.
Add sodium hydroxide solution (NaOH) dropwise to the test tube.
If a yellow precipitate forms, it indicates the presence of a methyl ketone (2-pentanone).
If no precipitate forms, it suggests the presence of an ethyl ketone (3-pentanone).
Pentanol and 3-Pentanone differentiation:
Conduct the iodoform test by adding iodine solution to a test tube.
Add sodium hydroxide solution (NaOH) dropwise to the test tube.
Since both compounds lack a methyl ketone group, there will be no formation of a yellow precipitate. Therefore, the iodoform test cannot differentiate between pentanol and 3-pentanone.
1-Propanol and 2-Propanol differentiation:
The iodoform test is not applicable here since both compounds lack a methyl ketone group. Therefore, the test cannot be used to differentiate between 1-propanol and 2-propanol.
For more questions like Pentanone click the link below:
https://brainly.com/question/14003512
#SPJ11
A 10,0-L cylinder of gas is stored at room temperature (20.0°C) and a pressure of 1800 psi. If the gas is
transferred to a 6.0-L cylinder, at what temperature in CELCIUS would it have to be stored in order for the
pressure to remain at 1800 psí? Reminder, convert your temperature to Kelvin before you begin the problem.
(Please put units)
Answers
Considering the Charles' law, the gas would have a temperature of -109.2 C.
Charles' lawFinally, Charles' law establishes the relationship between the volume and temperature of a gas sample at constant pressure. This law says that the volume is directly proportional to the temperature of the gas. That is, if the temperature increases, the volume of the gas increases, while if the temperature of the gas decreases, the volume decreases.
Charles' law is expressed mathematically as:
\(\frac{V}{T} =k\)
If you want to study two different states, an initial state 1 and a final state 2, the following is true:
\(\frac{V1}{T1} =\frac{V2}{T2}\)
Temperature of the gas in this caseIn this case, you know:
P1= 1800 psiV1= 10 LT1= 20 C= 293 K (being 0 C= 273 K)P2= 1800 psiV2= 6 LT2= ?You can see that the pressure remains constant, so you can apply Charles's law.
Replacing in the Charles's law:
\(\frac{10 L}{293 K} =\frac{6 L}{T2}\)
Solving:
\(\frac{10 L}{293 K} T2=6 L\)
\(T2=\frac{6 L}{\frac{10 L}{293 K} }\)
T2=163.8 K= -109.2 C
The gas would have a temperature of -109.2 C.
Learn more about Charles's law:
https://brainly.com/question/4147359?referrer=searchResults
Jodie is studying the sleeping habits of various types of animals. She wants to know how long each type of animal sleeps during the day.
Jodie puts each of the animals in a cage with food, water, and light. However, she cannot stay to watch the animals all day.
Which of the following tools would be most helpful to Jodie's experiment?
A.
a tape recorder
B.
a video camera
C.
a metric ruler
D.
a triple beam balance
Answers
Terry and James are partners in a mystery lab. The boys have a compound light microscope and several unlabeled slides. Their task is to find out everything they can about the samples on the slides. Terry puts a slide on the microscope stage and focuses the lenses on the sample. He can see that the sample is made up of tiny cells.
Even without knowing anything else about the cells he sees, what can Terry reasonably conclude about them?
Answers
From the samples on the slide made up of tiny cells, Terry can conclude that the cells were produced by other cells.
How does cell production occur?Cell production occurs often in a human protein, such as yeast, bacteria, or mammalian cells in culture, which then start producing the protein in large quantities. A new organism is created during the process of splicing a gene into a production cell.
Cells are often produced from other cells by the process of replication. All living things, from microorganisms to humans, rely on cells for structure and function. Scientists regard them as the tiniest form of life. Cells contain the biological machinery that produces the proteins, chemicals, and signals that are responsible for everything that occurs within our bodies.
Learn more on cell production here: https://brainly.com/question/23117462
#SPJ1
Ethylene glycol (C2H6O2) is used as an antifreeze in cars. If 400 g of ethylene glycol is added to 4.00 kg of water, what is the molality? Calculate how much the freezing point of water will be lowered. The freezing-point depression constant for water is Kf = –1.86°C/m.
Answers
Answer:
The freezing point of water will be lowered 3°C
Explanation:
Remember that molality is generally the standard for calculating the concentration, between molarity and molality. In this question we have to apply molality, given the units as moles and kilograms.
We can start by receiving the molality of this solution through the following formula...
\(\mathrm{m}=\mathrm{(mass}\mathrm{/molar\:mass)} * (1000/\mathrm{mass\:of\:solvent)},\)
\(\mathrm{m} =\mathrm{(400.0 g / 62.07 g/mol) * (1000 / 4000.0 g)},\)
\(\mathrm{m} = (400/62.07) * (1000 / 4000) = \left(\frac{400}{62.07}\right)\left(\frac{1000}{4000}\right) = \frac{1000}{620.7} = 1.61108\dots\mathrm{m}\)
Now we can determine how much the freezing point of water will be lowered, given that the freezing-point depression constant for water is Kf= –1.86°C/m. Remember that " Kf " is the final temperature, so to determine how much the temperature of the water will decrease, we can note the change in temperature...
\(\mathrm{change\:in\:temperature} = ( - 1.86\mathrm{C/m})(1.61\mathrm{m}) = - 1.86 * 1.61 = -2.9946\)
\(-2.9946 = \mathrm{About\:-3C}\)
Hence the freezing point of water will be lowered 3°C.
How can we increase the rate of collisions between the reactants in this reaction?
Mg + 2HCl → MgCl2 + H2
A.
Increase the concentration of H2 in the reaction mixture.
B.
Decrease the temperature of the reactants in the reaction mixture.
C.
Increase the concentration of Mg in the reaction mixture.
D.
Decrease the temperature of the entire reaction mixture.
E.
Decrease the concentration of HCl in the reaction mixture.
Answers
Answer: C. Increase the concentration of Mg in the reaction mixture.
Explanation: I got this right on Edmentum.

What mass of iron should be produced if 11. 0g of aluminum react with 30. 0g of iron (III) oxide?
Answers
The mass of iron should be produced if 11. 0g of aluminum reacts with 30. 0g of iron (III) oxide is 10.50 g.
To determine the mass of iron produced, we need to use stoichiometry and the balanced chemical equation for the reaction between aluminum and iron(III) oxide.
The balanced chemical equation is:
2 Al + \(Fe_{2} O_{3}\) → + 2 Fe
From the equation, we can see that 2 moles of aluminum react with 1 mole of iron(III) oxide to produce 1 mole of iron.
First, we need to determine the limiting reactant by comparing the number of moles of aluminum and iron(III) oxide.
Moles of aluminum = mass of aluminum / molar mass of aluminum
= 11.0 g / 26.98 g/mol (molar mass of aluminum)
= 0.407 mol
Moles of iron(III) oxide = mass of iron(III) oxide / molar mass of iron(III) oxide
= 30.0 g / 159.69 g/mol (molar mass of iron(III) oxide)
= 0.188 mol
Since the stoichiometric ratio of aluminum to iron(III) oxide is 2:1, we can see that 0.188 mol of iron(III) oxide requires 0.376 mol of aluminum. However, we have only 0.407 mol of aluminum, which is in excess.
Therefore, the limiting reactant is iron(III) oxide. The amount of iron produced is determined by the moles of iron(III) oxide used. Moles of iron = 0.188 mol (same as moles of iron(III) oxide)
Now we can calculate the mass of iron produced using its molar mass (55.85 g/mol):
Mass of iron = Moles of iron × Molar mass of iron
= 0.188 mol × 55.85 g/mol
= 10.50 g
Therefore, the mass of iron produced is approximately 10.50 grams.
Know more about the Balanced chemical equation here:
https://brainly.com/question/13451900
#SPJ8
What is the difference between sigma and pi bonds in valence bond theory? what orbitals are involved in making these bonds?.
Answers
The overlapping of atomic orbitals distinguishes sigma and pi covalent bonds. When atomic orbitals overlap, covalent bonds form.
The way atomic orbitals overlap affects bond properties such as bond length, bond angle, and bond enthalpy. This overlap occurs in two ways, resulting in two types of covalent bonds: sigma bonds and pi bonds.
This type of covalent bond is formed by head-on positive (same phase) overlap of atomic orbitals along the internuclear axis. Sigma bonds are the strongest covalent connections due to the direct overlapping of the orbitals involved. The electrons in a bond are frequently referred to as electrons.
In this state, one half-filled p orbital from each participating atom overlaps head-on along the internuclear axis.
Pi bonds are generated by the sideways positive (same phase) overlap of atomic orbitals parallel to the internuclear axis. The axes of the atomic orbitals are parallel to each other during bond formation, while the overlapping is perpendicular to the internuclear axis.
Learn more about atomic bonds at
https://brainly.com/question/27950782?referrer=searchResults
#SPJ4
The density of Lead is 11.4 g/ml. If a sample of Lead has a mass of 91 g, what is the
volume of this sample of Lead?
Answers
Answer:
Volume of Lead = 7.98 ml³
Explanation:
Given:
Density of Lead = 11.4 g/ml
Mass of Lead = 91 g
Find:
Volume of Lead
Computation:
Volume = Mass / Density
Volume of Lead = 91 / 11.4
Volume of Lead = 7.98 ml³
which example is an exothermic reaction?responses ammonium nitrate dissolving in water ammonium nitrate dissolving in water concentrated hydrochloric acid dissolving in water concentrated hydrochloric acid dissolving in water ammonium chloride dissolving in waterammonium chloride dissolving in watersugar dissolving in water
Answers
An exothermic reaction is the chemical reaction which releases heat to the surroundings. Among the options you provided, the example of an exothermic reaction is ammonium nitrate dissolving in water. Option A is correct.
This reaction is exothermic because the dissolution process releases heat to the surroundings, as well as the temperature of the solution will increases.
The other examples you provided, such as the dissolving of concentrated hydrochloric acid, ammonium chloride, and sugar in water, are all exothermic processes as well, but they are not chemical reactions, as no new substances are formed. They are simply physical changes, where the substances dissolve in the solvent.
Hence, A. Ammonium nitrate dissolving in water is the correct option.
To know more about exothermic reaction here
https://brainly.com/question/28546817
#SPJ4
--The given question is incorrect, the correct question is
"Which example is an exothermic reaction? A) ammonium nitrate dissolving in water B) concentrated hydrochloric acid dissolving in water C) sugar dissolving in water D) ammonium chloride dissolving in water."--
Answer the following question in the attachment please for homework that is due tomorrow

Answers
Answer:
Details on attachment.
Explanation:
See attached worksheet.

Why do quartz and glass both have very high melting points?
please can someone give an explanation
Answers
Covalent bonding is present in Quartz. Covalent bonds result in a high melting point. Covalent solids are insoluble in most solvents.
Why does quartz have such a high melting point?Quartz is very hard to melt because quartz is unsteady above 870 Celsius, and molten silica is wobbling below 1713 Celsius. In the interval between 870 and 1713 degrees, quartz tends to change to tridymite or cristobalite, not melt. It is hard to heat quartz to melt, in the region of 1650 Celsius
Melting point: The melting point of quartz is higher than 1700°C. Curie temperature for alpha and beta quartz: The Curie warmth for quartz is 573°C.
So we can conclude that Crystal quartz has a very particular melting point. Because quartz glass is a single component it doesn't form eutectics
Learn more about melting points here: https://brainly.com/question/40140
#SPJ1
Which of the three subatomic particles; ____1______ ALWAYS the same, ____2_____changes in isotopes, and _____3____ changes in ions?
Answers
2- Neutrons change in isotopes
3- Electrons change in ions
How many moles of butane do we have if we have 5.50 x 10^24 molecules of butane (C4H10)?
Answers
9.14moles
Explanations:According to the Avogadro's constant,
\(1mole\text{ of }C_4H_{10}=6.02\times10^{23}molecules\)We are to determine the moles of 5.50 x 10^24 molecules of butane. This is expressed as:
\(\begin{gathered} mole\text{ of }C_4H_{10}=\frac{5.50\times10^{24}}{6.02\times10^{23}} \\ mole\text{ of C}_4H_{10}=0.914\times10^{24-23} \\ mole\text{ of C}_4H_{10}=0.914\times10 \\ mole\text{ of C}_4H_{10}=9.14moles \end{gathered}\)Therefore the required mole of butane is 9.14moles
Suppose 3.40 g of iron(ity lodide is dissolved in 100 ml of a 0.20 Maqueous solution of silver nitrate Calculate the final malarity of iodide anion in the solution. You can assume the volume or the solution doesn't change when the iron(II) fodide is dissolved in it Round your answer to significant digit.
Answers
When 4.50 g of iron iodide is dissolved in 100 ml of a 0.2M aqueous solution of silver nitrate, the final molarity of iodide anion is 0.415M
We have to first obtain the number of moles of iron iodide (FeI₂)
Molar mass of FeI₂= 310 g/mol
So, number of moles of FeI₂ = 3.40/310 = 0.011 mol
Volume of solution = 100mL or 0.1L
Concentration of AgNO₃ = 0.20 M
number of moles of AgNO3 = 0.20M x 0.1L = 0.02 mol
The balanced equation of the reaction is
2AgNO₃ + FeI₂ -------> 2AgI + Fe(NO₃)₂
Amount of excess FeI₂ = 0.0525 - 0.011 = 0.0415mol
Therefore, Concentration of excess iodide in solution
= Concentration of excess FeI₂/volume of solution
= 0.0415mol /0.1
=0.415 M
To know more about molarity here
https://brainly.com/question/15056656
#SPJ4
a solution is prepared by dissolving 99.7 g of csi in enough water to form 895 ml of solution. calculate the mass % of the solution if the density of the solution is 1.06 g/ml.a solution is prepared by dissolving 99.7 g of csi in enough water to form 895 ml of solution. calculate the mass % of the solution if the density of the solution is 1.06 g/ml.12.7.5%9.4.9.3%
Answers
The mass percent of the solution is approximately 10.51%.
To calculate the mass percent of the solution, we need to determine the total mass of the solution.
The mass of the solution can be calculated using the density and volume of the solution:
Mass of the solution = Density × Volume
Mass of the solution = 1.06 g/ml × 895 ml
Mass of the solution = 948.7 g
The mass percent of the solution can be calculated by dividing the mass of the solute (CSI) by the mass of the solution and multiplying by 100:
Mass percent = (Mass of CSI / Mass of the solution) × 100
Mass percent = (99.7 g / 948.7 g) × 100
Mass percent ≈ 10.51%
Learn more about mass here
https://brainly.com/question/11954533
#SPJ11
Sabendo que os calores de combustão do enxofre monoclínico e do enxofre rômbico são, respectivamente, - 297,2 kJ/mol e - 296,8 kJ/mol, calcule a variação de entalpia na transformação de 1 mol de enxofre rômbico em enxofre monoclínico. S (mon.) + O2(g) SO2(g) ∆H1 = –297,2 kJ/mol S (rômb.) + O2(g) SO2(g) ∆H2 = –296,8 kJ/mol
Answers
Responda:
+ 0,9kJ / mol
Explicação:
Dados os calores de combustão do enxofre monoclínico e enxofre rômbico como - 297,2 kJ / mol e - 296,8 kJ / mol, respectivamente para a variação na transformação de 1 mol de enxofre rômbico em enxofre monoclínico conforme mostrado pela equação;
S (mon.) + O2 (g) -> SO2 (g)
Uma vez que são todos 1 mol cada, a mudança na entalpia será expressa como ∆H = ∆H2-∆H1
Dado ∆H2 = -296,8kJ / mol
∆H1 = -297,2kJ / mol
∆H = -296,8 - (- 297,2)
∆H = -296,8 + 297,2
∆H = 297,2-296,8
∆H = + 0,9kJ / mol
Portanto, a mudança na entalpia da equação é + 0,9kJ / mol
If a liquid contains 60% sugar and 40% water throughout its composition then what is it called? Homogeneous mixture Solvent Heterogeneous mixture Compound
Answers
The liquid described, containing 60% sugar and 40% water throughout its composition, is called a homogeneous mixture.
A homogeneous mixture is a type of mixture where the components are uniformly distributed throughout the mixture, resulting in a uniform composition and appearance. In a homogeneous mixture, the components are not visibly distinct and cannot be easily separated by physical means, such as filtering or settling.
Homogeneous mixtures can be either single-phase or multi-phase, depending on the number of components present and the nature of their interactions. Examples of homogeneous mixtures include solutions (such as saltwater), alloys (such as brass), and some types of gels and emulsions.
Visit here to learn more about homogeneous mixture brainly.com/question/30587533
#SPJ11
This diagram would represent the enthalpy changes in which
example?
O endothermic synthesis reaction
Oa melting solid
O a boiling liquid
O a hot flask after mixing two chemicals
Answers
An endothermic reaction is a melting solid
What is an enthalpy change?Enthalpy (H) is a thermodynamic state function that accounts for the internal energy of a system plus the work done by or on the system. When a chemical reaction takes place or a physical change occurs, there may be a change in the enthalpy of the system, represented as ΔH (delta H), which is the difference between the enthalpy of the products and the enthalpy of the reactants.
If ΔH is negative, it means that the reaction or process releases heat energy, and is therefore exothermic. If ΔH is positive, it means that the reaction or process absorbs heat energy, and is therefore endothermic. Enthalpy change is usually measured in joules (J) or kilojoules (kJ) per mole of substance involved in the reaction or process.
Learn more about endothermic reaction:https://brainly.com/question/23184814
#SPJ1
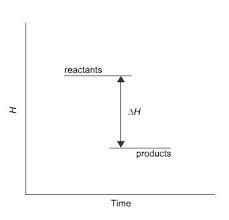
Answer:
A hot flask after mixing two chemicals.
65g of NaCl are placed in a beaker and enough water is added to fill the beaker to 1 liter. What is the molar out of this solution? Round answer to the nearest hundredths
Answers
The molarity of the solution is 1.11 M (rounded to 2 decimal places). To find the molarity (M) of the solution, we need to know the number of moles of NaCl and the volume of the solution in liters.
How do molality and molarity differ?Although a solution's molarity is determined by the moles of solute divided by the volume of the solution in litres, a solution's molality is determined by the moles of solute divided by the mass of the solvent in kilogrammes.
The number of moles of NaCl can be calculated using its molar mass and the given mass of NaCl:
Number of moles = mass / molar mass
The molar mass of NaCl is 58.44 g/mol (22.99 g/mol for Na + 35.45 g/mol for Cl).
Number of moles of NaCl = 65 g / 58.44 g/mol = 1.1138 mol (rounded to 4 decimal places)
The volume of the solution is given as 1 liter.
Now we can use the formula for molarity:
Molarity (M) = number of moles / volume (in liters)
M = 1.1138 mol / 1 L = 1.1138 M (rounded to 2 decimal places)
To know more about molarity visit:-
brainly.com/question/8732513
#SPJ1
how do i find the atomic mass and number ?
Answers
To get the atomic mass you need to combine the neutrons and protons together, and the atomic number is the number of protons in an atom!
Hope this helps if not comment below please!!!
A thermite reaction releases large amounts of heat and light, resulting in the melting of the iron metal that forms during the reaction.
fe2o3 + 2al → al2o3 + 2fe
determine the correct mole ratio for iron (iii) oxide and aluminum based on the balanced chemical equation. fe2o3:al =
Answers
Answer:
The mole ratio for iron (iii) oxide to aluminium is
1:2
20) When ice melts, what happens to the water molecules?
Answers
Answer:
start moving faster
Explanation:
i would guess because they slow down our freeze when they get cold
Explain how sulfur forms its ion.
Answers
Explanation:
the sulfur ato is in group 6 of the periodic table. In order to become an ion the ato needs to gain 2 electrons so it has a full outer shell of electron and is stable. so the atom S goes to S2+ + 2e
Help!! Need it asap thankyou!

Answers
Answer:
ano gagawin dyan? HAAHAHA
A. Fill in the blanks to complete the idea of the paragraph.
1.______________ is anything that occupies space and has 2. ______________. It has many properties and characteristics. It also exists in different phases, namely, 3.___________such as rocks and metals, 4.____________ such as water and soda, and 5. ___________ such as air.
B. Is it solid , liquid or gas? Write your answer on the space provided for.
_______________6. flower
_______________7. water vapor
_______________8. lotion
_______________9. smoke
______________10. keyboard
C. List at least 5 properties of matter.
11. _____________
12. ____________
13. ____________
14. ____________
15. ____________
Answers
Answer:
1. Matter
2. Weight
3. Solid
4. Liquid
5. Gas
6. Solid
7. Gas
8. Liquid
9. Gas
10. Solid
11. Density
12. Mass
13. Volume
14. Temperature
15. Boiling point
Which of the following is the best definition of a scientific theory?
A.
a broad, well-supported explanation of related observations or events
B.
a description of a natural phenomenon that is always observed to occur under specific conditions
C.
a highly tentative statement about the natural world that is likely to be significantly changed or discarded
D.
a plausible answer to a scientific question that is used to guide research
Answers
Answer:
A. A broad, well-supported explanation of related observations or events
Explanation:
A scientific theory is a detailed reasoning of a well supported observation or experiment. Though, not just anyone can create a scientific theory... it has to be widely accepted throughout the scientific community!
A law on the other hand, is simply a description of things that happen in the natural world. It doesn't quite say why, with a law, it just is what it is! Laws don't become theories and theories do not become laws!
I hope I was of assistance! Science can be difficult! Have a nice day and spread the love <3
suppose the radius of an atom in a simple cubic unit cell is 0.17 nm. what is the edge length of the unit cell in nm.
Answers
Suppose the radius of an atom in a simple cubic unit cell is 0.17 nm. 0.34 nm is the edge length of the unit cell.
In a simple cubic unit cell, the atoms are arranged in a cube with one atom at each corner. The distance from the center of an atom to the edge of the cube is half of the edge length. Therefore, the edge length of the unit cell can be calculated as twice the radius of the atom:
A body-centered cubic unit cell has one atom in the centre and atoms at each of the cube's four corners. We must know the separation between atoms situated at the opposite corners of the cube in order to calculate the edge length of the unit cell.
Think of a diagonal line joining the cube's two opposed corners while traversing its centre. The hypotenuse of a right triangle with two sides equal to the edge length of the unit cell might be imagined as being this diagonal line.
\(Edge length = 2radius\)
Edge length = 2 x 0.17 nm
Edge length = 0.34 nm
So the edge length of the simple cubic unit cell is 0.34 nm.
Learn more about edge length here
https://brainly.com/question/32077959
#SPJ11