What is the state of matter if the particles are spread out and have little to no inter-molecular forces?
Answers
The state of matter in which particles are immobile and ordered in a repeating pattern is called a solid.
In a solid, the particles are closely packed together and held in a fixed position by strong intermolecular forces. This makes the solid rigid and unable to flow, which is why solids have a fixed shape and volume.
The repeating pattern of particles in a solid is what gives it its characteristic crystal structure. This structure can be seen in minerals, metals, and many other types of solids.
The ordered arrangement of particles in a solid also gives it unique physical and chemical properties, such as high density, low compressibility, and high melting and boiling points.
For more questions like Solids click the link below:
https://brainly.com/question/13324776
#SPJ11
The state of matter where the particles are spread out and have little to no inter-molecular forces is gas.
What is matter?Matter is defined as anything that occupies space and has a mass. It is present in various forms, such as solid, liquid, gas, and plasma, and can change from one form to another with the application of heat or pressure. It is also composed of tiny particles known as atoms and molecules, which have specific properties that determine their behavior when subjected to different conditions.
The state of matter is dependent on how the particles of matter are arranged and how they interact with one another. There are three states of matter, which are solid, liquid, and gas. When the particles of matter are tightly packed, the matter is in the solid state, while the particles of matter are loosely packed and move around freely when the matter is in the liquid state.
When the particles of matter are spread out and have little to no inter-molecular forces, the matter is in the gaseous state. In this state of matter, the particles are free to move about in all directions, colliding with one another and the walls of their container. The inter-molecular forces that exist in a gas are extremely weak, and as a result, the particles are not held together in a rigid structure.
Learn more about Matter here: https://brainly.com/question/3998772
#SPJ11
Related Questions
Hemispheres are north or south of the equator. What season is it in Asia when it is winter here?
Answers
Answer:
Summer
Explanation:
Assuming you are in the northern hemisphere, it would be summer.
Asia is in the southern hemisphere and because of the Earth's axis of 23 degrees, it causes seasons to be inversed in the other hemisphere.
Hope that helps
what is the percent abundance of carbon-12 and carbon-13 if carbon-12 has a mass 12.00 amu, carbon-13 has a mass of 13.001, and the average atomic mass is 12.010?
Answers
The percent abundance of carbon-12 and carbon-13 is 2.08%
The average atomic mass can be calculated by multiplying the percent abundance by the atomic mass for each isotope and then adding up the values. Here's the formula:
Average atomic mass of carbon = (¹²C percent abundance)(¹²C atomic mass) + (¹³C percent abundance)(¹³C atomic mass)
In the problem known as an atomic mass value, carbon-12 has a move ss 12.0carbonbon-13 has a mass of 13.001 and the average atomic mass is 12.010. so that:
Average atomic mass of carbon = (x) (¹²C atomic mass) + (x) (¹³C atomic mass)
12.010 = x (12.00) + x (13.001)
12.010 = 12.00x + 13.001x
12.010 = 25.001x
x = 25.001/12.010 = 2.08%
So, the percent abundance of carbon-12 and carbon-13 is 2.08%
Learn more about the average atomic mass of carbon here https://brainly.com/question/24666479
#SPJ4
Consider the equation and check all statements that apply to it: HA ↔ H+ + A−
(Select Apply Question)
A. It represents a strong acid buffer system.
B. It represents a weak acid buffer system.
C. Adding a strong acid would shift the equilibrium to the left
D. Adding a strong acid would shift the equilibrium to the right
Answers
Answer:
The answer is B & C
Explanation:

Which of the following best predicts how the partial pressures of the reacting species will be affected if a small amount of Arlg) 8. is added to the equilibrium mixture at constant volume? a. PN02 will-decrease and PN204 will increase b. Pwo will increase and PN204 will decrease oth PNo2 and PN204 will decrease. No change will take place.
Answers
When an inert gas, such as argon (Ar), is added to a reaction mixture at constant volume, it behaves independently of the chemical reaction occurring. Inert gases do not participate in the reaction or undergo any chemical changes themselves. Instead, they simply occupy space within the container.
Since the addition of argon does not affect the concentrations or partial pressures of the reacting species, it does not cause any changes in the equilibrium position. The reaction will continue to reach and maintain its equilibrium state with the same partial pressures of the reacting species as before the addition of the inert gas.
Therefore, in the given scenario, the partial pressures of the reacting species, such as P(NO2) and P(N2O4), will remain unchanged when a small amount of argon gas is added to the equilibrium mixture at constant volume.
To know more about chemical refer here
https://brainly.com/question/29240183#
#SPJ11
What is the reaction to . Na2SO3 + 2 HC2H3O2 → H2SO3 + 2 NaC2H3O2
Answers
what is the empirical formula for a compound if a sample contains 1.0 g of S and 1.5 g of O?
1.SO
2.SO3
3.S202
4. S203
Answers
How many o atoms are in 259 g of c12h21o9? molar mass of c12h21o9 = 309.29 g/mol
Answers
The number of atoms in 259 g of C₁₂H₂₁O₉ is 45 x 10²³ oxygen.
What is molar mass?The molar mass of many compounds can be calculated by dividing the mass of the compound by the number of moles of the compound.
Given the molar mass is 309.29 g/mol
Given weight= 259g
number of moles = mass / molar mass
259g / 309.29g/mol =0.837mol
1 mol contains 6.022 x 10²³ molecules
0.837 mol contain 6.022 x 1023 x 0.837 = 5.0 x 10²³ molecules
1 molecule of C₁₂H₂₁O₉ contains 9 oxygen atoms
5.0 x 10²³ molecules of C₁₂H₂₁O₉ contain 9x 5 x 10²³ oxygen atoms.
=45 x 10²³ oxygen atoms
Thus, the number of atoms in 259 g of C₁₂H₂₁O₉ is 45 x 10²³ oxygen atoms
To learn more about molar mass, refer to the link:
https://brainly.com/question/22997914
#SPJ4
Answer:
Explanation: your the best
what do you mean by Arsenic Pollution?
Answers
Arsenic contamination of groundwater is a form of groundwater pollution which is often due to naturally occurring high concentrations of arsenic in deeper levels of groundwater.Arsenic contamination of ground water is found in many countries throughout the world, including the US.
Write the balanced equation for the ionization of the weak base pyridine, c5h5n , in water, h2o.
Answers
The equation for the ionization of the weak base pyridine in water is:
C5H5N + H2O ⇌ C5H5NH+ + OH-
Explanation:
Pyridine is a weak base, so it will react with water to create hydroxide (OH-) ions, along with the conjugate acid of pyridine, which is called pyridinium ion (C5H5NH+). The overall equation for the reaction can be written as:C5H5N + H2O ⇌ C5H5NH+ + OH-In this equation, the arrows indicate that the reaction is reversible, meaning that the products can also react to form the reactants. Therefore, the concentration of each species in the reaction mixture will be related by an equilibrium constant (K).
The balanced equation for the ionization of the weak base pyridine in water is C5H5N + H2O ⇌ C5H5NH+ + OH-.
To know more about ionization visit:
brainly.com/question/1602374
#SPJ11
Potassium metal reacts with chlorine gas to form solid potassium chloride. Answer the following:
Write a balanced chemical equation (include states of matter)
Classify the type of reaction as combination, decomposition, single replacement, double replacement, or combustion
If you initially started with 78 g of potassium and 71 grams of chlorine then determine the mass of potassium chloride produced.
Answers
The balanced chemical equation between pottasium and chlorine is as follows: 2K + Cl₂ → 2KCl. It is a combination reaction.
What is a chemical reaction?A chemical reaction is a process, typically involving the breaking or making of interatomic bonds, in which one or more substances are changed into others.
According to this question, a chemical reaction occurs between potassium metal and chlorine gas to form pottasium chloride as follows:
2K + Cl₂ → 2KCl
The chemical reaction is a combination reaction because it involves the combination of two elements to form a compound.
Learn more about chemical reaction at: https://brainly.com/question/22817140
#SPJ1
explain how you could use ir spectroscopy to differentiate between compounds f and g. (b) explain how you could use ir spectroscopy to differentiate between compounds d and e. (c) if you wanted to distinguish between compounds b and f, would it be more suitable to use ir spectroscopy or mass spectrometry? explain. (d) would mass spectrometry be helpful for distinguishing between compounds a and d? explain.
Answers
Since these two substances are both alcohols, IR spectroscopy cannot be used to separate them.
These two substances may be distinguished from one another using mass spectrometry because they have various molecular weights.The IR spectroscopy theory is based on the idea that molecules have a tendency to absorb particular light frequencies that are unique to the corresponding structure of the molecules.The electromagnetic spectrum's infrared section, which includes light with a longer wavelength and lower frequency than visible light, is the subject of infrared spectroscopy (IR spectroscopy).
Learn more about IR spectroscopy here:
https://brainly.com/question/29441785
#SPJ4
The formula for lysergic acid is C6H6N,O2. What is the percent composition of carbon in the acid?
10.44%
71.62%
4.48%
139.61%
27.92%
Answers
Given that the formula for lysergic acid is C6H6N,O2. The percent composition of carbon in the acid is 71.62%.
Given that the formula for lysergic acid is C6H6N,O2.To calculate the percent composition of carbon in the acid, we need to find the molar mass of carbon and the molar mass of lysergic acid.We know that the molar mass of carbon is 12.01g/molMolar mass of Lysergic Acid = (6 x molar mass of carbon) + (6 x molar mass of hydrogen) + (1 x molar mass of nitrogen) + (2 x molar mass of oxygen)Molar mass of Lysergic Acid = (6 x 12.01 g/mol) + (6 x 1.01 g/mol) + (1 x 14.01 g/mol) + (2 x 16.00 g/mol)Molar mass of Lysergic Acid = 268.3 g/molNow, we can calculate the percent composition of carbon in lysergic acid.
Percent Composition of Carbon in lysergic acid = (Number of Carbon atoms x Molar mass of Carbon atoms) / (Total Molar mass of lysergic acid)Percent Composition of Carbon in lysergic acid = (6 x 12.01 g/mol) / 268.3 g/molPercent Composition of Carbon in lysergic acid = 0.267Percent Composition of Carbon in lysergic acid = 26.7 %So, the percent composition of carbon in the acid is 71.62%.
To know more about lysergic acid visit:
https://brainly.com/question/7139742
#SPJ11
The enthalpy of vaporization for methanol is 35.2 kJ/ mol. Methanol has a vapor pressure of 1 atm at 64.7 °C. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 41.9 °C?
Give your answer in atmospheres, to the third decimal point.
Question 2
3 pts
The enthalpy of vaporization for dimethyl ether is 27.5 kJ/mol. Dimethyl ether has a vapor pressure of 760 torr at 34.6 °C. Using the Clausius-Clapeyron equation, what is the vapor pressure tor methanol at 0.1 °C? Give your answer in torr, to the first decimal point.

Answers
Answer:
To use the Clausius-Clapeyron equation, we need to know two sets of conditions for the substance in question. Let's start with question 1:
Question 1:
Given:
Enthalpy of vaporization, ΔHvap = 35.2 kJ/mol
Vapor pressure at T1 = 1 atm (or 760 torr), T1 = 64.7°C
We want to find: Vapor pressure at T2 = 41.9°C
First, we need to convert temperatures to Kelvin:
T1 = 64.7 + 273.15 = 337.85 K
T2 = 41.9 + 273.15 = 315.05 K
Now we can use the Clausius-Clapeyron equation:
ln(P2/P1) = -ΔHvap/R * (1/T2 - 1/T1)
where P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively, R is the gas constant (8.314 J/mol·K), and ln is the natural logarithm.
Solving for P2, we get:
P2/P1 = e^(-ΔHvap/R * (1/T2 - 1/T1))
P2 = P1 * e^(-ΔHvap/R * (1/T2 - 1/T1))
Substituting the given values, we get:
P2 = 1 atm * e^(-35.2 kJ/mol / (8.314 J/mol·K) * (1/315.05 K - 1/337.85 K))
P2 = 0.496 atm
Rounding to three decimal places, the vapor pressure of methanol at 41.9°C is 0.496 atm.
Answer: 0.496 atm
Question 2:
Given:
Enthalpy of vaporization, ΔHvap = 27.5 kJ/mol
Vapor pressure at T1 = 760 torr, T1 = 34.6°C
We want to find: Vapor pressure at T2 = 0.1°C
Converting temperatures to Kelvin:
T1 = 34.6 + 273.15 = 307.3 K
T2 = 0.1 + 273.15 = 273.25 K
Using the Clausius-Clapeyron equation:
ln(P2/P1) = -ΔHvap/R * (1/T2 - 1/T1)
Solving for P2, we get:
P2/P1 = e^(-ΔHvap/R * (1/T2 - 1/T1))
P2 = P1 * e^(-ΔHvap/R * (1/T2 - 1/T1))
Substituting the given values, we get:
P2 = 760 torr * e^(-27.5 kJ/mol / (8.314 J/mol·K) * (1/273.25 K - 1/307.3 K))
P2 = 7.25 torr
Rounding to one decimal place, the vapor pressure of dimethyl ether at 0.1°C is 7.3 torr.
Answer: 7.3 torr
When Juan got home from school, he noticed a foul smell coming from the kitchen. He decided to investigate the smell and found fruit sitting on the counter. There was evidence that a chemical reaction had occurred. When he looked at the fruit, he saw that it had turned brown and was soft to the touch. Write a short claim evedence and reasoning. If you could separate them it would be geat
Answers
Answer:
See explanation
Explanation:
A chemical reaction is said to have occurred when there is a permanent, irreversible change in a substance.
Considering the fruit in the question, the changes in the fruit can not be reversed. Nothing can ever be done to return the fruit to its original form. This ultimately shows that a chemical reaction has taken place.
Secondly, the occurrence of a chemical reaction can be confirmed by evolution of a gas. The foul smell that filled the kitchen is the gas evolved during the chemical reaction involving the rotten fruit.
2. A 20-year-old woman goes to the Emergency Department due to symptoms of palpitations, dizziness, sweating, and paresthesia that have not resolved over the past several days. Her history suggests an anxiety disorder, and blood gases and electrolytes are ordered. Her doctor prescribes a benzodiazepine after a positron emission tomography (PET) scan shows increased perfusion in the anterior end of each temporal lobe. Which of the following blood gases would be expected at the time of admission of this patient?
A. pH 7.51; Pa co: 49 mm Hg: [HCO3] = 38 mEq/L; Anion Gap - 12 mEq/L
B. pH 7.44; Pa co2-25 mm Hg; [HCO3] = 16 mEq/L; Anion Gap = 12 mEq/L
C. pH 7.28: Pa coz 60 mm Hg: [HCO3] =26 mEq/L; Anion Gap = 12 mEq/L
D. pH 7.28: Pa co2 20 mm Hg: [HCO3] = 16 mEq/L: Anion Gap = 25 mEq/L
E. pH 7.51: Pa co2 20 mm Hg: [HCO3] = 24 mEq/L; Anion Gap = 12 mEq/L
Answers
The expected blood gas values for this patient at the time of admission of patient is option E. pH 7.51; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 24 mEq/L; Anion Gap = 12 mEq/L
A 20-year-old woman presents to the Emergency Department with persistent symptoms of palpitations, dizziness, sweating, and paresthesia. She has a history suggestive of an anxiety disorder.
To assess her condition, blood gases and electrolytes are ordered, and a positron emission tomography (PET) scan is performed. The PET scan reveals increased perfusion in the anterior portion of each temporal lobe. Based on these findings, the doctor prescribes a benzodiazepine medication.
The expected blood gas values at the time of admission can be determined by analyzing the given options:
A. pH 7.51; PaCO₂ = 49 mm Hg; [HCO₃]⁻ = 38 mEq/L; Anion Gap = 12 mEq/L
B. pH 7.44; PaCO₂ = 25 mm Hg; [HCO₃]⁻ = 16 mEq/L; Anion Gap = 12 mEq/L
C. pH 7.28; PaCO₂ = 60 mm Hg; [HCO₃]⁻ = 26 mEq/L; Anion Gap = 12 mEq/L
D. pH 7.28; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 16 mEq/L; Anion Gap = 25 mEq/L
E. pH 7.51; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 24 mEq/L; Anion Gap = 12 mEq/L
By evaluating the options, the most appropriate choice is:
E. pH 7.51; PaCO₂ = 20 mm Hg; [HCO₃]⁻ = 24 mEq/L; Anion Gap = 12 mEq/L
This option presents a higher pH (alkalosis) and a decreased PaCO₂ (respiratory alkalosis), which could be consistent with the patient's symptoms of hyperventilation due to anxiety. The [HCO₃]⁻ level within the normal range and a normal anion gap further support this interpretation.
In summary, the expected blood gas values for this patient at the time of admission are a higher pH, decreased PaCO₂, normal [HCO₃]⁻, and a normal anion gap, indicative of respiratory alkalosis likely caused by hyperventilation related to her anxiety disorder.
To know more about Blood gas values here: https://brainly.com/question/27826544
#SPJ11
IF YOU ANSWER CORRECTLY U WILL RECIVE BRAINLEIST AND 10 pts! AsAp! thank youuu u will make my day by answering CoRrEcTlY!
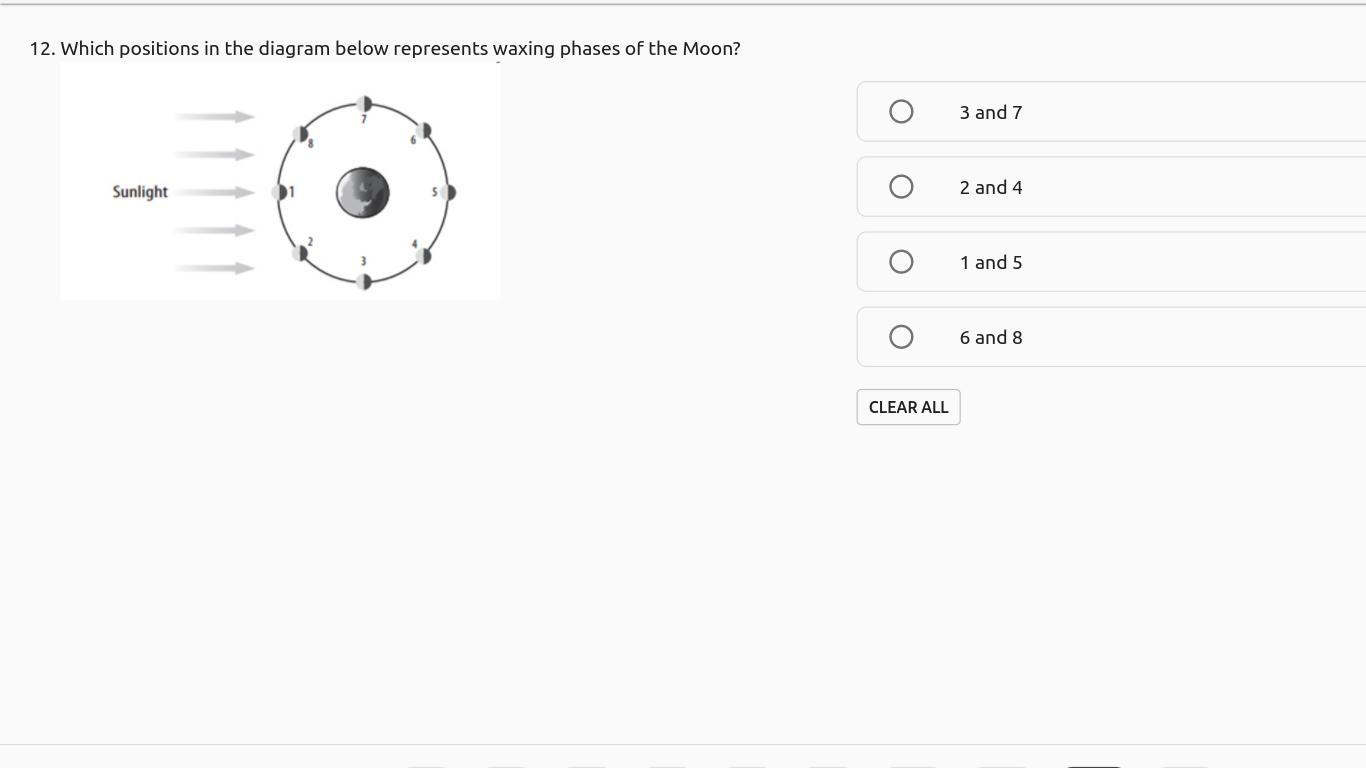
Answers
Answer:
6 and 8
Explanation:
an atom with 3 protons that loses an electron becomes…

Answers
Answer:
A +1 Ion of Lithium
Explanation:
Lithium has the atomic number of 3, therefore it has 3 protons
An electron has a negative charge, so losing an electron gives an atom a positive charge.
Answer:
Positive 1 ion of lithium
Explanation:
hope this helps!
Explain what is meant by the natural abundance of isotopes. Can someone help me to answer this plz
Answers
What happens to the dry ice after sometime?
Answers
Answer:
As a rough rule, five pounds of dry ice will turn from a solid to a gas in 24 hours. It's best to pick up the block of ice just a few hours before your party so it's as frozen as possible when the bash starts.
Explanation:
What did you learn about factors that affect the speed of melting ice? Explain your answer with evidence, such as your data and observations.
pls help
Answers
The factors that can affect the speed of melting ice include the presence of wind, the level of humidity in the surrounding air, and the amount of sunlight or other heat sources in the area.
Temperature, surface area, and the presence of materials like salt are just a few of the variables that might influence how quickly ice melts. Ice will often melt more quickly at higher temperatures because the heat energy causes the ice molecules to vibrate and disintegrate. Because there is more surface area exposed to the environment, increasing the surface area of the ice by breaking it into smaller pieces or smashing it can also speed up the melting process. The pace of melting can also be impacted by the addition of chemicals like salt to ice. Ice melts at a lower temperature than it would otherwise because salt lowers the freezing point of water when it is added to it. Here is why salt is often used to melt ice on roads and sidewalks during winter. Overall, the speed of melting ice can be influenced by a variety of factors, and the specific conditions in a given situation will determine how quickly the ice will melt.
For more such questions on heat
https://brainly.com/question/29419715
#SPJ11
what is the mass of a substance whose density is 7.86 g/ml and volume is 6.5ml
Answers
Answer:
Multiply the density by the volume
Since density is at g/mL you don't need to convert volume into L and just multiply it as mL
7.86g/mL x 6.5mL = 51.09g
Therefore the mass of the substance is 51.09g
A small balloon at standard temperature has a volume of 80.0mL. When placed into a Dewar flask of liquid nitrogen (-200C), it contracts. What is the balloon’s new volume?
Answers
Since the temperature is directly proportional to volume, the new volume of the gas is 21.4 mL.
We have the following information;
Initial temperature(T1) = 273 K
Initial volume (V1) = 80.0mL
Final temperature (T2)= -200°C + 273 = 73 K
Final volume (V2) = ?
Using the relation of Charles' law;
V1/T1 = V2/T2
V1T2 = V2T1
V2 = V1T2 /T1
V2 = 80.0mL × 73 K/ 273 K
V2 = 21.4 mL
Learn more: https://brainly.com/question/2192784
Guided
Learning
ar Inequalities in One Variable - Item 7601
Pre-Quiz
Practice
from a, it will be greater than b.
Post-Quiz
Finish
If the ordered pair (a, b) satisfies the inequality y> x-4, three of these statements are
true. Which statement is NOT true?
a and b may be equal to each other.
We
Answers
The statement about the inequality that is NOT true is (A), a and b may be equal to each other.
How to determine true statements?This is because the inequality y> x-4 means that y must be greater than x-4. If a and b are equal, then y = x-4, which means that the inequality is not satisfied.
The other three statements are true because:
If 4 is subtracted from a, it will be greater than b because y> x-4 means that y must be greater than x.
If 4 is subtracted from b, it will be less than a because y> x-4 means that y must be greater than x.
If 4 is subtracted from both a and b, the inequality will still be true because y> x-4 means that y must be greater than x, even if x and b are both decreased by 4.
Therefore, the answer to the question is the statement a and b may be equal to each other.
Find out more on inequality here: https://brainly.com/question/25275758
#SPJ1
Complete question:
Guided Learning ar Inequalities in One Variable - Item 7601
Pre-Quiz
Practice
from a, it will be greater than b.
Post-Quiz
Finish
If the ordered pair (a, b) satisfies the inequality y> x-4, three of these statements are true. Which statement is NOT true?
a and b may be equal to each other.
If you subtract 4 from a, it will be greater than b.
If you subtract 4 from b, it will be less than a.
If you subtract 4 from both a and b, the inequality will still be true.
- Jed was told to put some containers in one of the cold stores at work.
The labels read 'Store below -5°C'. There are two store rooms. One is
kept at 15°F and one at 25°F. Which one should he choose?
Answers
Answer:
15 because it is the closest
Explanation:
Which contains more atoms, a pound of lithium (Li) or a pound of lead (Pb)?
Answers
A pound of lithium contains more atoms than a pound of lead due to its lower atomic mass. Lithium has an atomic mass that is almost 30 times less than lead, so it takes a larger number of lithium atoms to make up the same mass as lead atoms.
The number of atoms in a substance is determined by its atomic mass, which is the sum of the masses of all the protons, neutrons, and electrons in an atom. Lithium has an atomic mass of 6.941 atomic mass units (amu), while lead has an atomic mass of 207.2 amu. Therefore, one pound of lithium will contain more atoms than one pound of lead.
To calculate the number of atoms in a pound of each substance, we need to use Avogadro's number, which is 6.022 * 10^{23} atoms per mole. One mole of lithium weighs 6.941 grams, while one mole of lead weighs 207.2 grams. Therefore, one pound of lithium (453.59 grams) is equivalent to 65.33 moles, or 3.93 * 10^{25} atoms. On the other hand, one pound of lead is equivalent to 2.43 moles, or 1.46 * 10^24 atoms.
learn more about lead Refer: brainly.com/question/25046469
#SPJ11
For each solution, calculate the initial and final pH after add adding 0.010 mol of HCl. a. 500.0 mL of pure water b. 500.0 mL of a buffer solution that is 0.125 M in HC_2H_3O_2 and 0.115 M in NaC_2H_3O_2 c. 500.0 mL of a buffer solution that is 0.155 M in C_2H_5NH_2 and 0.145 M in C_2H_5NH_3Cl
Answers
a. Pure Water:
HCl will react with water to form H3O+ and Cl- ions:
HCl + H2O → H3O+ + Cl-
Since the water is initially neutral, the initial concentration of H3O+ is 1.0 x 10^-7 M. Adding 0.010 mol of HCl to 500.0 mL of water will result in a final volume of 500.010 mL (assuming negligible volume change upon addition of HCl). Thus, the final concentration of H3O+ is:
[H3O+] = moles HCl / final volume in L
[H3O+] = 0.010 mol / 0.500010 L
[H3O+] = 0.00002 M
pH = -log[H3O+]
Initial pH = -log(1.0 x 10^-7) = 7.00
Final pH = -log(0.00002) = 4.70
b. Buffer Solution of HC2H3O2 and NaC2H3O2:
The buffer solution contains both a weak acid (HC2H3O2) and its conjugate base (C2H3O2-), which will resist changes in pH upon the addition of an acid or a base. The initial pH of the buffer solution can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([base]/[acid])
The pKa of acetic acid (HC2H3O2) is 4.76. Substituting the given concentrations, the initial pH of the buffer solution is:
pH = 4.76 + log(0.115/0.125)
pH = 4.74
When HCl is added, it reacts with the weak base (C2H3O2-) in the buffer solution to form the weak acid (HC2H3O2) and Cl- ions:
HCl + C2H3O2- → HC2H3O2 + Cl-
The change in concentration of the weak acid and its conjugate base can be calculated using the balanced equation and the initial concentrations of the buffer components:
moles of HC2H3O2 formed = moles of HCl added
moles of C2H3O2- consumed = moles of HC2H3O2 formed
moles of NaC2H3O2 remaining = moles of C2H3O2- remaining
[HCl] = 0.010 mol / 0.500 L = 0.020 M (concentration of added HCl)
[HC2H3O2] = 0.125 M (initial concentration of weak acid)
[C2H3O2-] = 0.115 M (initial concentration of conjugate base)
[NaC2H3O2] = 0.115 M (initial concentration of salt)
Using an ICE table (Initial, Change, Equilibrium) to calculate the final concentrations:
Initial:
[HC2H3O2] = 0.125 M
[C2H3O2-] = 0.115 M
[NaC2H3O2] = 0.115 M
Change:
[HC2H3O2] = 0.125 M + 0.010 mol / 0.500 L = 0.145 M
[C2H3O2-] = 0.115 M - 0.010 mol / 0.500 L = 0.095 M
[NaC2H3O2] = 0.115 M -
brainly.com/question/31255541
#SPJ11
What process does the body use for transporting broken down molecules?
Answers
The process used by the body for transporting broken-down molecules is known as passive transport.
This process involves the movement of molecules in and out of cells without the use of energy or the need for ATP. Passive transport occurs through a variety of mechanisms, including diffusion, osmosis, and facilitated diffusion.
In diffusion, molecules move from an area of higher concentration to an area of lower concentration until the concentration of the molecules is equal.
Osmosis is the diffusion of water molecules through a selectively permeable membrane from a high concentration of water to a low concentration of water.
Facilitated diffusion is the process by which molecules move through protein channels in the cell membrane. This process does not require the use of energy and is a type of passive transport.
To learn more about passive transport
https://brainly.com/question/13542102
Predict whether each of the following molecules is polar or nonpolar: (a) IF, (b) CS2, (c) SO3, (d) PCl3, (e) SF6, (f) IF5.
Answers
The polarity status of the molecules are as follows;
IF - nonpolar CS₂ - nonpolar SO₃ - nonpolarPCl₃ - polar SF₆ - nonpolar IF₅ - polarWhat is polarity?Polarity is the dipole-dipole intermolecular forces between the slightly positively-charged end of one molecule to the negative end of another or the same molecule.
A polar molecule has difference in electronegativity values. For example; all the three chlorine atoms pull the electrons from the phosphorous atom making it a polar molecule in PCl₃.
Also, iodine pentafluoride (IF₅) is a polar molecule because the central iodine (I) atom in IF₅ is surrounded by five fluorine (F) atoms forming a square pyramidal shape.
Learn more about polarity at: https://brainly.com/question/1946554
#SPJ1
What vale is represented by the symbol Mr
Answers
the answer for this question is Mister
(the following problems involve using the gas laws.) 1. a 7.89 liter sample of gas has a pressure of 850 torr. what volume will it occupy if the pressure is changed to 457 torr? (760 torr
Answers
The changed pressure according to Charles law is 14.723l.
What is Charles law ?
Charles' law asserts that a gas's volume is equal to a constant number times the temperature of that gas, as determined by the Kelvin scale (zero Kelvin corresponds to -273.15 degrees Celsius).
What is pressure ?
The SI unit for pressure, the pascal (Pa), which equals one newton per square metre (N/m2) of surface area, is the measure of pressure. An object's pressure is inversely proportional to its area under which it is being forced and is directly proportional to the force it generates.
PV= nrT
PV/T=K
so, P1 V1/ T1 = P2 V2/T2
=P1 V1 = P2V2 (Boyles law)
data given,
V1= 7.89l
P1= 850 for r
P2= 457 for r
V2 = ?
850*7.89=457*V2
V2=850*7.89/457= 14.6750l
According to the charles law
V propotional to T
V1/T1 = V2/T2
=6.50/298= V2/675
V2=6.50/298*675=14.723l
Therefore, the changed pressure according to Charles law is 14.723l.
Learn more about Charles law from the given link.
https://brainly.com/question/14842720
#SPJ4