What is the Scientific
Method?
Answers
Answer:
I just finished a unit on Scientific Method in my science class! Anyway, it's defined in the screenshots below. Hope this helps!


Related Questions
108Xe is expected to be stable. Why is this false?
Answers
When the structure of 108Xe with S=0 is calculated, it has been noted that it contains 54 protons and 54 neutrons with strong symmetry, therefore 108Xe was thought to be stable, but when its half life is determined experimentally, it comes to be just 58 microseconds, which is truly quite low.
Xenon is a chemical element with the symbol Xe and the atomic number 54. It is a colourless, odourless noble gas found in tiny amounts in the Earth's atmosphere. Dizziness, nausea, vomiting, loss of consciousness, and death can result from excessive inhalation. Poor judgement, confusion, or loss of consciousness can all lead to death, preventing self-rescue.
Xenon is used in several specialised light sources. It emits a gorgeous blue light when activated by an electrical discharge. Xenon lamps are used in a wide range of applications, such as high-speed electronic flash bulbs for photographers, sunbed lamps, and bactericidal lights for food preparation and processing. Xenon improves long-term cognitive function in mice, reduces neuronal loss and chronic neuroinflammation, and improves survival after traumatic brain injury.
To learn more about Xenon, here
https://brainly.com/question/5516586
#SPJ4
which tissue carrys messages to and from the brain
Answers
Answer:
nervous tissues
Explanation:
Nervous tissue is found in the brain, spinal cord, and nerves. It is responsible for coordinating and controlling many body activities. ... The cells in nervous tissue that generate and conduct impulses are called neurons or nerve cells. These cells have three principal parts: the dendrites, the cell body, and one axon
credits to the person who provided this info.
Write a 4-7 sentence summary on the topic Radiation. (don't mind the orange color)

Answers
Answer:
pa brainliest answer po pa heart pa follow me and back
Explanation:
SUMMURY
Radiation is energy. It travels in the form of energy waves or high-speed particles. Radiation can occur naturally or be man-made. ... Ionizing radiation, which includes ultraviolet radiation, radon, x-rays, and gamma rays.
Radiation is energy that comes from a source and travels through space at the speed of light. This energy has an electric field and a magnetic field associated with it, and has wave-like properties. You could also call radiation “electromagnetic waves”.
DIFFENITION :
radiation
1a: the action or process of radiating
b: the process of emitting radiant energy in the form of waves or particles
c(1): the combined processes of emission, transmission, and absorption of radiant energy
(2): the transfer of heat by radiation
— compare CONDUCTION, CONVECTION
2a: something that is radiated
b: energy radiated in the form of waves or particles
3: radial arrangement
4: ADAPTIVE RADIATION
Q
1 At which electrode does reduction always take place?
2 Give the half-equation for the reaction at the anode, during
the electrolysis of these molten compounds:
a potassium chloride b calcium oxide
3 Give the two half-equations for the electrolysis of:
a a concentrated solution of hydrochloric acid, HCl
b a dilute solution of sodium nitrate, NaNO3
c a dilute solution of copper(II) chloride, CuCl2
Answers
how is an electron orbital similar to a parabola?
Answers
Answer:
Explained below.
Explanation:
First of all, the orbital path of electron is mostly parabolic in electric field.
In an electric field, electrons behave very similar to a projectile. Thus, Electrons have a parabolic path in an electric field simply because the speed of the electrons in a direction which is perpendicular to the electric field is constant since there is no force. Therefore, there will be no acceleration along that perpendicular direction. However there will be an acceleration that is constant in the direction of the electric field which makes it act in a similar manner to a projectile under gravity.
Which factor decide the reactivity of alkyl halide?
Answers
Answer:
The reactivity order reflects both the strength of the C-X bond and the stability of X(-) as a leaving group and leads to the general conclusion that alkyl iodides are the most reactive members of this functional class
who is credited with the discovery of atomic number?
Answers
Henry Moseley used the analysis of X-ray spectra to determine the atomic number in the year 1913. He discovered that when the atomic number of an element is increased by one, certain lines in its x-ray spectra travel by the same amount each time.
In 1913–1914, English physicist Henry Moseley discovered and published the law. The "atomic number" of an element was previously only known as its position in the periodic table and had no known connection to any quantifiable physical property before Moseley's work. Chemical elements are identified exclusively by their atomic number. We owe Henry Moseley, a British physicist, credit for this discovery since he used physical rules to support this empirical and chemical understanding of the atomic number.
Learn more about atomic number here:
https://brainly.com/question/16858932
#SPJ4
pls help what is 56+98 i ned help plesss
Answers
Answer:
What is 56 + 98?
= 154Explanation:
You're welcome.
Part 1. Matching
Place the letter for the correct element category from the list below next to each description.
Part 2. Fill in the blank
Write the word or phrase that best completes each sentence. Choose from the following:
group, period, atomic number, periodic, metal, metalloid, nonmetal, chlorine, magnesium.
1. The elements on the periodic table are arranged in order of increasing _____________________.
2. The arrangement of the elements on the periodic table shows a predictable, repeating, ____________
pattern that allows us to predict chemical properties.
3. The elements Na, Mg, and Cu are each classified as ___________________ elements.
4. When elements are located in the same ______ they exhibit similar chemical and physical properties.
5. The element ________ is an example of an element that is classified as a nonmetal.
Part 3. Classifying
For each electron configuration identify the element and decide which group the element belongs to on the
periodic table. (Hint: Find the total number of electrons for each and refer to page 186 in your Student
Book to determine the element and group number.
Electron configuration Element Group number
1. 1s2
2s22p6.
2. 1s2
2s22p6
3s2.
3. 1s2
2s2
2p6
3s2
3p6
.
4. 1s2
2s2
2p6
3s2
3p6
4s2
.
5. 1s2
2s22p6
3s23p3.
A NATURAL APPROACH TO CHEMISTRY
_______
_______
_______
_______
_______ _______
_______
_______
_______
_______
1. ___ The element sodium (Na) belongs to this group of elements.
2. ___ These elements are not likely to chemically bond with other elements.
3. ___ These elements are located in the center of the periodic table.
4. ___ These elements tend to gain one electron to fill their outer shell and are sometimes colored gases.
5. ___ These elements have two electrons in their highest unfilled energy level.
Answers
Answer:
Try asking a friend or teacher
Explanation:
helps better than this site because alot of the answers are different even though they might of had the same test
An abandoned Indiana coal mine spoil bank (wastes) contains chunks of pyrite minerals. Under constant erosion and weathering, the pyrites leach large amounts of sulfuric acid (H2SO4). The spoil banks are also mixed with large quantities of basic limestone and clay carbonates. What should occur over time?
Answers
Answer:
The acid will be neutralized overtime
Explanation:
The presence of the pyrites leads to the leaching of large amounts of sulphuric acid, however the basic carbonates neutralizes the acid according to the reaction equation;
CaCO3 + H2SO4 ---> CaSO4 + CO2 + H2O.
This will prevent all the deleterious consequences associated with the leaching of the acid in the abandoned coal mine.
How are the properties of metals and non-metals different?
Answers
Answer:
Explanation:
Properties of metals:
1. They have a lustre ( shine )
2. They have a silvery-grey or golden-yellow colour.
3. They conduct heat and electricity.
4. They are ductile ( can be drawn into wires ) .
5.They are malleable ( can be hammered into thin sheets ).
6. They are sonorous ( make a ringing sound when hit ).
Properties of non-metals:
1. They display a variety of colours.
2. They are poor conductors of heat and electricity.
3. They are not lustrous, ductile or malleable.
Hope it helps
plz mark as brainliest!!!!!!
Are the elements potassium (k) and chlorine (cl) likely to form a chemical bond?
Answers
Potassium (K) and chlorine (Cl) are likely to form a chemical bond. The combination of these elements can result in the formation of an ionic bond, leading to the creation of potassium chloride (KCl), a stable compound commonly known as table salt.
Potassium (K) and chlorine (Cl) are elements from Group 1 and Group 17 of the periodic table, respectively. They have a tendency to form chemical bonds due to their electron configurations. Potassium has one valence electron in its outermost energy level, while chlorine has seven valence electrons. In order to achieve a stable electron configuration, chlorine requires one additional electron, while potassium needs to lose one electron. This electron transfer can occur between the two atoms, resulting in the formation of an ionic bond. As a result, the potassium atom donates one electron to the chlorine atom, creating a positively charged potassium ion (K+) and a negatively charged chloride ion (Cl-). These oppositely charged ions are then attracted to each other, forming a strong ionic bond. The resulting compound, potassium chloride (KCl), is a crystalline solid with a regular arrangement of alternating potassium and chloride ions. This compound is stable and commonly found as table salt in our daily lives.
Learn more about Potassium (K) here:
brainly.com/question/28794999?
#SPJ11
Which atom in the ground state has a stable electron configuration?
A) carbon
B) magnesium
C) krypton
D) oxygen
Answers
Answer:
A. carbon
Explanation:
500 mL D5W is infusing at a rate of 25 macrogtt / min . The drop factor is 10 gtt /m L. How long will it take for the IV to infuse ?
Answers
500 mL D5W is infusing at the rate of 25 macrogtt / min. The drop factor is 10 gtt /m L and it will take 20 minutes for the IV to infuse.
First, we need to calculate the flow rate in mL/min using the given information.
25 macrogtt/min × 10 gtt/mL = 250 gtt/mL
The flow rate in mL/min is the same as the number of drops per minute divided by the drop factor, which is:
250 gtt/mL ÷ 10 gtt/mL = 25 mL/min
Now we can use the formula:
time = volume ÷ rate
where volume is the total volume to be infused and rate is the flow rate.
Plugging in the given values, we get:
time = 500 mL ÷ 25 mL/min
time = 20 minutes
To know more about drop factor here
https://brainly.com/question/26275156
#SPJ4
Please can somebody give me the correct answers.please be realigned
I will be so grateful!!

Answers
Answer: I hope this helps :
An element in Group 5 = Bismuth (Bi)
A halogen = Fluorine (F) or Astatine (At)
An alkali Metal = Lithium (L)
A metal in Group 6 = Selenium (Se) , Tellurium (Te) , Polonium (Po)
A gas made up of individual atoms = Argon (Ag)
An element that forms 1+ ions = Lithium
The most reactive element in Group 1 = francium (it doesn't appear in the image)
The most reactive element in Group 7 = Fluorine
An element that is a good catalyst= Iron (Fe) Cobalt (Co) , Nickel (Ni)
An element that does not react with anything = Argon
A metal that floats on water = Lithium
An element with a full outer energy of electrons = Helium (He), neon (Ne), and argon (Ar)
A transition Metal = Iron (Fe) Cobalt (Co) , Nickel (Ni)
A noble gas = Argon (Ar)
The element in Group 6 , Period 5 = Molybdenum , Tellurium
A non-metal = Fluorine , Argon
A gas made up of Diatomic molecule = Argon (Ar)
An element that forms 1- ions =
The Group 1 element with the highest melting point = Lithium
The Group 7 element with the highest boiling point = Astatine (As)
An element with 3 electrons in it's outer energy level = Boron
An element that forms coloured compounds = Iron
An element that has a coloured vapour = Chlorine Fluorine
A metal that can form ions with different charges = Iron, Cobalt , Lead
Explanation:
Halogen : Are any of the six nonmetallic elements that make up Group 17 of the periodic table e.g fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At).
All elements in Group 1 are Alkali metals( except hydrogen)
Examples :lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen)
Argon is one of the inert gases that normally exist as single atoms.
Transition metals are good metal catalysts because they easily lend and take electrons from other molecules. A catalyst is a chemical substance that, does not affect the thermodynamics of a reaction but increases the rate of reaction.
Transition metals ; Scandium. Titanium. Vanadium. Chromium. Manganese. Iron. Cobalt. Nickel.
Noble gases(inert gases) don't react with anything . The family of noble gases includes helium, neon, argon, krypton, xenon, and radon.
Lithium is the lightest metal and has density about half of water.
Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell.
chlorine, fluorine, carbon, nitrogen, arsenic, phosphorus, selenium are examples of non-metal.
Noble gases : helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn).
The following 5 element gases are found as diatomic molecules at room temperature and pressure:
Hydrogen – H. ...
Nitrogen – N. ...
Oxygen – O. ...
Fluorine – F. ...
Chlorine – Cl.
Lithium, Li melts at 180°C.
From the lowest boiling and melting point to the highest, the group in order is fluorine, chlorine, bromine, iodine and astatine.
Like other transition metals, iron forms coloured compounds. The table shows some examples of these. Note that iron can form two different ions in its compounds. Iron(II) compounds contain the Fe 2+ ion and iron(III) compounds contain the Fe 3+ ion.
Elements in group seven(Halogens) : As you move down the group the halogens become darker in colour. For example fluorine is a very pale yellow whereas iodine will be dark purple in colour when it is in a vapour state.
A few elements, all metals, can form more than one possible charge. For example, iron atoms can form 2+ cations or 3+ cations. Cobalt is another element that can form more than one possible charged ion (2+ and 3+), while lead can form 2+ or 4+ cations.
What, approximately, is the highest concentration of co2 measured in the earth's atmosphere in modern times?
Answers
In modern times the highest concentration of \(CO_{2}\) measured in the earth's atmosphere is 400 ppm.
The concentration of the \(CO_{2}\) which is also refereed as global annual is increased by 50%.The rise in concentration of the \(CO_{2}\) start from 280 ppm during \(10^{4}\) years up to the mid-18th century to 421 ppm as of May 2022.The reason behind the rise of concentration of the \(CO_{2}\) is the Industrial Revolution.The concentration of the \(CO_{2}\) is rises in such pace the it is very dangerous for human health and environment.Learn about global annual
https://brainly.com/question/2921854
#SPJ4
a 1.42 g sample of a pure compound, with formula m2so4 was dissolved in water and treated with an excess of aqueous calcium chloride, resulting in the precipitation of all the sulfate ions as calcium sulfate. the precipitation was collected, dried, and found to weigh 1.36 g. determine the atomic mass of m, and identify m
Answers
The atomic mass of M is 95.94 g/mol, and M is Barium (Ba) regarding a 1.42 g sample of a pure compound, with formula m2so4.
The balanced chemical equation for the reaction between M2SO4 and CaCl2 is:
M2SO4(aq) + CaCl2(aq) -> CaSO4(s) + 2MCl(aq)From the equation, we can see that 1 mole of M2SO4 reacts with 1 mole of CaSO4, so the number of moles of M2SO4 in the original sample is equal to the number of moles of CaSO4 precipitated:
moles of M2SO4 = moles of CaSO4 = mass of CaSO4 / molar mass of CaSO4molar mass of CaSO4 = 136 g/mol (40.08 g/mol for Ca + 32.06 g/mol for S + 4x16.00 g/mol for O)moles of M2SO4 = 1.36 g / 136 g/mol = 0.01 molThe molar mass of M2SO4 can be calculated using the mass of the sample and the number of moles:
molar mass of M2SO4 = 1.42 g / 0.01 mol = 142 g/molSince the formula for M2SO4 contains two M atoms, the atomic mass of M is half of the molar mass of M2SO4:
atomic mass of M = 142 g/mol / 2 = 71 g/molLooking at the periodic table, the only element with an atomic mass close to 71 g/mol is Barium (Ba). Therefore, M is Barium (Ba).
Learn more about Pure Compounds:
https://brainly.com/question/28540199
#SPJ4
how do Newton's laws of motion describe when and how objects move?
Answers
A clinical trial was conducted to test the effectiveness of a drug for treating insomnia in older subjects. Before treatment, 16 subjects had a mean wake time of 104.0 min. After treatment, the 16 subjects had a mean wake time of 94.1 min and a standard deviation of 23.7 min. Assume that the 16 sample values appear to be from a normally distributed population and construct a 99% confidence interval estimate of the mean wake time for a population with drug treatments. What does the result suggest about the mean wake time of 104.0 min before the treatment? Does the drug appear to be effective? Construct the 99% confidence interval estimate of the mean wake time for a population with the treatment. min<μ
Answers
The mean wake time of 104.0 min before treatment is outside the 99% confidence interval of the mean wake time after treatment, it suggests that the drug is effective. This is further confirmed by the significant decrease in the mean wake time after treatment of 94.1 min. Therefore, it can be concluded that the drug is effective in treating insomnia in older subjects.
A clinical trial was conducted to test the effectiveness of a drug for treating insomnia in older subjects. Before treatment, 16 subjects had a mean wake time of 104.0 min.
After treatment, the 16 subjects had a mean wake time of 94.1 min and a standard deviation of 23.7 min.
Assume that the 16 sample values appear to be from a normally distributed population and construct a 99% confidence interval estimate of the mean wake time for a population with drug treatments.
The formula for the confidence interval of the mean is:
\($$\overline{X} \pm z_{\alpha/2} \frac{s}{\sqrt{n}}$$\)
Here,
\($z_{0.005} = 2.576$\) for a 99% confidence interval as
\($α/2 = 0.005$\)
and the degrees of freedom is 15 since \($n-1=15$\).
Now, substituting all the values:
\($$94.1 \pm 2.576 \times \frac{23.7}{\sqrt{16}}$$\)
The calculation gives a 99% confidence interval estimate of the mean wake time of 94.1 ± 15.4 min (rounded off to one decimal place).
The mean wake time of 104.0 min before treatment is not within the 99% confidence interval of the mean wake time after treatment. This indicates that there is a significant decrease in the mean wake time after treatment.
Learn more about mean wake time from the given link:
https://brainly.com/question/16032908
#SPJ11
In the diagram below, what will allow more solute to be dissolved in the
solvent?
Answers
Answer:
missin a diagram buddy
Explanation:
four protons are initially held at the corners of a square that is 6.7 × 10-9 m on a side. they are then released from rest. what is the speed of each proton when the protons are very far apart?
Answers
The speed of each proton when they are very far apart is approximately 7.88 x 10⁵ m/s.
Assuming the protons are released from rest and experience only electrostatic repulsion from each other, we can use conservation of energy to find their final speeds when they are very far apart. At the initial configuration, the potential energy of the system is:
U_i = k_e × (q² / r) + k_e × (q² / r) + k_e × (q² / d) + k_e × (q² / d)
where k_e is the Coulomb constant, q is the charge of a proton, r is the distance between adjacent protons (i.e., the length of one side of the square), and d is the diagonal distance between opposite protons.
Substituting the values, we get:
U_i = (8.99e9 N m² / C²) × (1.6e-19 C)² × [(1 / 6.7e-9 m) + (1 / 6.7e-9 m) + (1 / 9.49e-9 m) + (1 / 9.49e-9 m)]
U_i = 8.77e-17 J
When the protons are very far apart, their potential energy approaches zero and their kinetic energy approaches the total energy of the system. Thus, we have:
U_f = K = 8.77e-17 J
where U_f is the final potential energy of the system, which is equal to the kinetic energy of the protons.
Since each proton has the same kinetic energy, we can write:
K = (1/2) × m × v²
where m is the mass of a proton and v is its final speed.
Solving for v, we get:
v = √(2 × K / m)
Substituting the values, we get:
v = √[(2 × 8.77e-17 J) / (1.67e-27 kg)]
v = 7.88e5 m/s
To know more about speed here
https://brainly.com/question/30462853
#SPJ4
What is specific gravity in minerals?
Answers
Specific gravity is the "heaviness" of a mineral. It is defined as a number that expresses the ratio between the weight of a mineral and the weight of an equal volume of water.
Answer:
The ratio of it's mass of an equal volume of water.
Explanation:
write the basic equilibrium equation for po4 3- besure to include the proper phases for all species within the reaction
Answers
The balanced equation for the dissociation of PO43- is given by;PO43-(aq) H2O(l) ⇌ HPO42-(aq) OH-(aq)The phosphate ion, PO43-, reacts with water to form HPO42- (monohydrogen phosphate) and OH- ions.
The chemical equilibrium between these species is represented by the equation above. In the dissociation reaction of PO43- the phosphate ion reacts with water (H2O) to form monohydrogen phosphate ion (HPO42-) and hydroxide ion (OH-). The reaction can be described as;PO43-(aq) H2O(l) ⇌ HPO42-(aq) OH-(aq)where the reactants are on the left and products on the right. In the equation, the state of matter of each reactant or product is written in parenthesis after its chemical formula. In this reaction, the reactant PO43- is an aqueous solution, while H2O is a liquid.
learn more about aqueous solution
https://brainly.com/question/19587902
#SPJ11
15
The diagram below shows a landscape
Upper atmosphere
Ocean
Where in the diagram would the air pressure be
the greatest
Answers
Answer:
No diagram no help
Explanation:
When two substances that cannot dissolve each other are mixed, a
mixture is formed.
A heterogeneous mixture that has very small dispersed particles and stays mixed for a long time is a
.
A heterogeneous mixture that has larger dispersed particles that settle over time is a
.
Answers
Answer:
The heterogeneous mixture that has very small dispersed particles and stays mixed for a long time is colloid. Because colloid has particles that are small enough to suspended but are as large that they can scatter light.
Answer:
1. heterogeneous
2. colloid
3. suspension
You have 45.6 mL of 10.1 M HBr. You need to use Mg(OH)₂ to neutralize it. How many grams of Mg(OH)2 are
necessary?
2HBr + Mg(OH)2 → 2H₂O + MgBr₂

Answers
Answer:
18.63 grams of Mg(OH)₂
Explanation:
2HBr(aq) + Mg(OH)₂(aq) → MgBr₂(aq) + 2H₂O(aq)
Since the stoichiometric ratio of the above equation is 2 : 1 : 1 : 2, therefore number of moles of HBr = 2 × number of moles of Mg(OH)₂. We can use the number of moles to find the mass present. [n=m/M].
To calculate the number of moles of hydrobromic acid present in the solution, we can multiply the concentration by the volume.
n(HBr) = 0.0456×10.1 = 0.46056 mol
∴ n(Mg(OH)₂) = 1/2 n(HBr) = 1/2 × 0.46056 = 0.23028 mol.
Now we have the number of moles of magnesium hydroxide required to neutralise the hydrobromic acid, we can use this value to calculate the mass present. Molar mass can be found using a standard IUPAC Periodic Table.
m(HBr) = nM = (0.23028)(1.008+79.90) = 18.63
Therefore, we require 18.63 grams of Mg(OH)₂ to neutralise 45.6 mL of 10.1 mol/L HBr.
Explain in a short sentence how you can tell if a reaction is a double replacement
reaction.
Answers
Explanation:
In a double displacement reaction, there is an actual exchange of partners to form new compounds.
The reaction is given as shown below:
AB + CD → AD + CB
One of the following conditions serves as the driving force for a double replacement reaction:
Formation of an insoluble compound or precipitateFormation of water or any other non-ionizing compoundLiberation of a gaseous product.how many valence electrons does tungsten have
Answers
Answer:74
Explanation:
Answer:
I believe Tungsten has 2 valence electrons.
Explanation:
Hope this helps!
Sulfur dioxide reacts with oxygen to produce sulfur trioxide. Write the equation. Identify the limiting reagent when 20.0 g of SO2 react with 15.6 g of O2.
Answers
The equation related to reaction between Sulfur dioxide and oxygen to produce sulfur trioxide is 2SO\(_2\)(g) + O\(_2\)(g) \(\rightarrow\) 2SO\(_3\) (g). SO\(_2\) is limiting reagent.
What is limiting reagent?The reactant that controls how much of the products are generated inside a chemical reaction is known as the limiting reagent. Since some of the other reactants remain that after limiting reagent has been used fully, it is occasionally discovered that they are in excess in the reactions. The theoretical yield is the most product that can theoretically be produced.
2SO\(_2\)(g) + O\(_2\)(g) \(\rightarrow\) 2SO\(_3\) (g)
moles of SO\(_2\) =20.0 g / 64.07
=0.312moles
moles of O\(_2\)= 15.6 g/32
=0.487moles
On dividing the moles by stoichiometry, out of O\(_2\) and SO\(_2\), SO\(_2\) is limiting reagent.
Therefore, the equation related to reaction between Sulfur dioxide and oxygen to produce sulfur trioxide is 2SO\(_2\)(g) + O\(_2\)(g) \(\rightarrow\) 2SO\(_3\) (g). SO\(_2\) is limiting reagent.
To know more about limiting reagent, here:
https://brainly.com/question/26905271
#SPJ1
how does one determine a molecular formula from the epirical formula
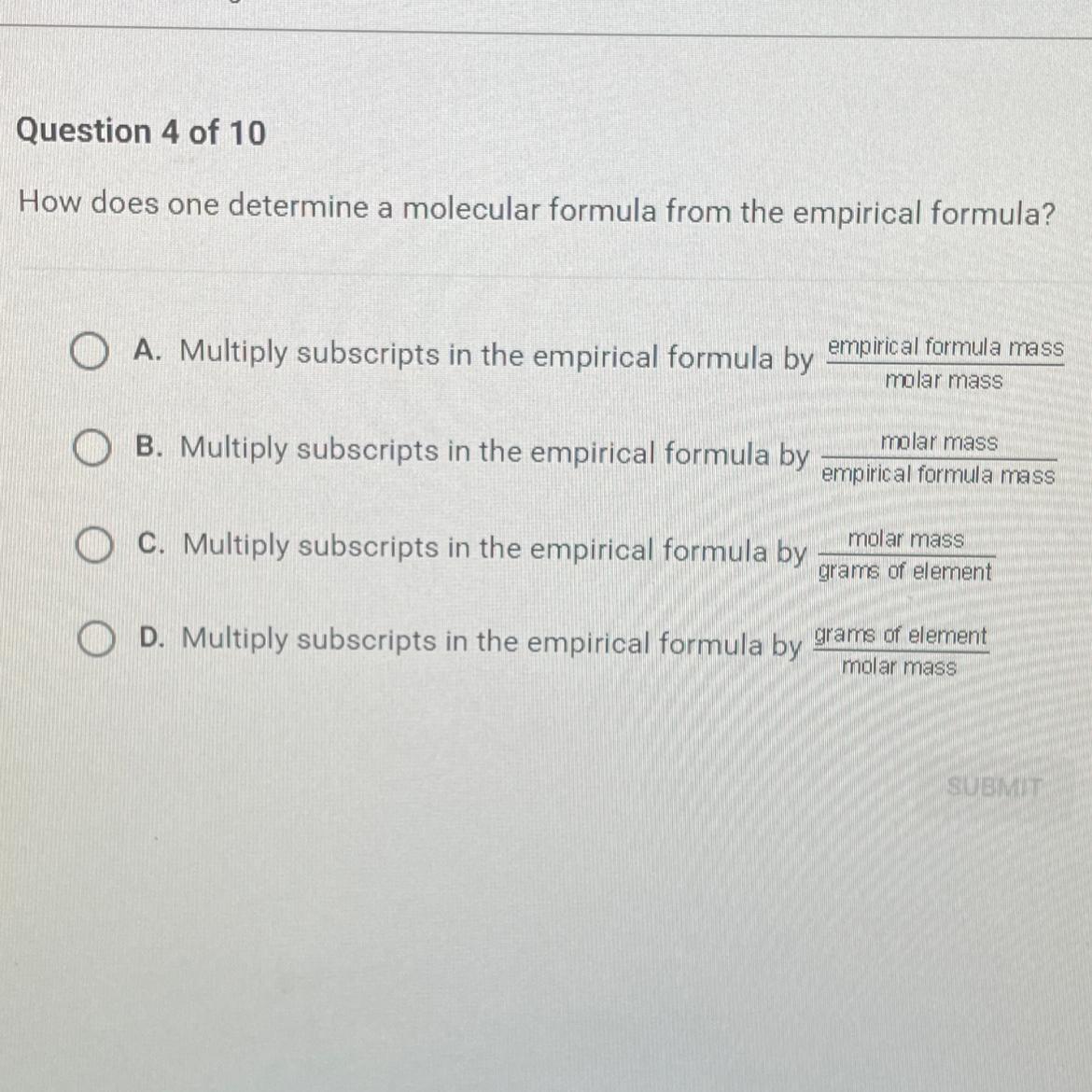
Answers
Answer:
C
Explanation: