what is the ph of a saturated solution of a metal hydrdoxide m(oh)3? ksp = 7.1e-12
Answers
The pH of a saturated solution of a metal hydroxide M(OH)₃ is approximately 12.00.
A saturated solution of a metal hydroxide M(OH)₃ has a pH that can be determined using the Ksp (solubility product constant) value provided, which is 7.1e-12. The Ksp expression for this metal hydroxide can be written as follows:
Ksp = [M³⁺][OH⁻]³
Since the solubility of M(OH)₃ in water is equal to the concentration of M³⁺ and OH⁻ ions in the saturated solution, let's represent the solubility as 's.' Therefore, the Ksp expression can be simplified as:
Ksp = (s)(3s)³
Solving for 's' will provide the concentration of hydroxide ions (OH⁻) in the solution:
7.1e-12 = (s)(27s⁴)
s⁵ = 2.63e-13
s = 1.01e-2
Now that we have the concentration of hydroxide ions, we can find the pOH and pH of the solution using the following formulas:
pOH = -log[OH⁻]
pH = 14 - pOH
Calculating the pOH:
pOH = -log(1.01e-2) ≈ 2.00
Now, finding the pH:
pH = 14 - 2.00 = 12.00
Therefore, the pH of the saturated solution of the metal hydroxide M(OH)₃ with a Ksp of 7.1e-12 is approximately 12.00.
To know more about pH, refer to the link below:
https://brainly.com/question/31604751#
#SPJ11
Related Questions
fill in the blank. "Properties of elements within a _________ on the periodic table change in a predictable way from one side of the table to the other"
Answers
Properties of elements within a period on the periodic table change in a predictable way from one side of the table to the other
From one side of the periodic table to the other, properties of elements within a period vary in a predictable manner. A horizontal row represents a period in the periodic table. The number of electron shells is the same for every atom in a row. Moving through a period causes elements to acquire electrons and protons and become less metallic. -Elements in the same period have the same number of electron shells.
As the atomic number rises, comparable features reoccur on a regular basis, which is reflected in this arrangement. From one side of the periodic table to the other, properties of elements within a blank shift in a predictable manner. those with comparable qualities are displayed in a column.
To know more about periodic table visit : https://brainly.com/question/11155928
#SPJ9
Urgent please please I really need help
I will give you 60 points just please help

Answers
Answer:
1 = -15. 2 = +5
Explanation:
i just did it good luck
How many grams of LiF are needed to make 87 g LiF solution into a
34.5% solution?
solution?
answer options:
30 g LIF
0.01 g LIF
45.8 g LIF
0.02 g LIF
Answers
Answer: 30 g LiF
Explanation:
a piece of charcoal is found to contain 30% of the carbon 14 that it originally had. when did the tree die from which the charcoal came? use 5600 years as the half-life of carbon 14
Answers
The time when the tree die from which the charcoal came is 1.02×10⁻⁴ years. if half-time of carbon-14 is 5600years.
An amount is dependent upon exponential decay on the off chance that it diminishes at a rate relative to its ongoing worth. Emblematically, this interaction can be communicated by the accompanying differential equation, where N is the amount and λ (lambda) is a positive rate called the dramatic rot steady, crumbling consistent, rate steady, or change consistent: dN/dt=-λN. The answer for this situation (see deduction beneath) is: N(t)=N₀e⁻\(\frac{k}{t}\), where N(t) is the amount at time t, N₀ = N(0) is the underlying amount, that is to say, the amount at time t = 0.
We know that exponential decay is given
A\(_t\) = A₀ e⁻\(\frac{k}{t}\) where K is decay constant.
We firstly find decay constant by using the half life-time
We know that t=ln2/k where t is half life time .Since,t=5600years given
=>5600=ln2/k
=>k=(ln2/5600)years
Now,it is given that at any particular instant amount of charcoal remain is 30 % of initial amount.
So, (30/100)A₀=A₀e⁻\(\frac{k}{t}\)
Here,k=(ln2/5600)years
=>30/100=e⁻ \(\frac{ln2/5600}{t}\)
Taking ln on both sides of equation,we get
=>ln(30/100)=ln(e⁻ \(\frac{ln2/5600}{t}\))
=>ln30-ln 100= - ln2/5600t
=>3.40 - 4.60 = -0.693/5600t
=> - 1.205 = -0.693/5600t
=>t=0.693/ (1.205×5600)
=>t=0.693 / 6748years
=>t=0.000102years or 1.02×10⁻⁴ years.
To know more about exponential decay,visit here:
https://brainly.com/question/14355665
#SPJ4
IWhich of the following solutions would be most likely to have the highest water concentration?
Multiple Choice hypertonic solution isotonic solution hypotonic solution water concentration and tonicity of a solution cannot be compared
Answers
"Hypotonic solution," refers to a solution with the highest water concentration due to its lower solute concentration compared to the other options, leading to water influx into cells.
A hypotonic solution would be most likely to have the highest water concentration. In a hypotonic solution, the solute concentration is lower than that inside the cell or compared to another solution. As a result, water tends to move into the cell or the solution to equalize the concentration.
When a cell is placed in a hypotonic solution, water molecules will move into the cell through the process of osmosis. This influx of water increases the water concentration inside the cell, leading to cell swelling or even bursting in extreme cases.
Compared to hypertonic and isotonic solutions, a hypotonic solution has a lower solute concentration, allowing for a higher concentration of water molecules. This results in a higher water concentration in the solution. It's important to note that the concept of tonicity is related to the relative solute concentrations between two solutions and their effect on cell osmosis. In this case, a hypotonic solution is characterized by a higher water concentration compared to the other options.
learn more about Hypotonic solution here:
https://brainly.com/question/28020628
#SPJ11
what is the final volume v2v2v 2 in milliliters when 0.523 ll of a 49.1 %% (m/v) solution is diluted to 20.6 %% (m/v)?
Answers
The final volume V2 in milliliters is
1246 ml
The volume percent of a solution is defined as the ratio of the volume of solute that is present in a solution, relative to the volume of the solution, as a whole.
We will use V1C1 = V2C2 to solve this problem.
V1 = 0.523 L = 523 mls
C1 = 49.1%
V2 = ? in mls
C2 = 20.6%
(523 ml)(49.1%) = (V2)(20.6%)
V2 = (523 ml)(49.1%)/(20.6%)
V2 = 1246 ml final volume
Hence, final volume v2 in milliliters is 1246 ml
Learn more about Volume here:
https://brainly.com/question/463363
#SPJ4
how is water vapour formed??If I donot think the
answer is suitable I will give bad ratings.
Answers
Water vapor is formed through a process called evaporation.
When water is heated, the molecules gain energy and become more active. This causes some of the water molecules to escape from the liquid phase and enter the gas phase, forming water vapor.
This process occurs naturally in various bodies of water, such as oceans, lakes, and rivers, as well as in plants through a process called transpiration.
Water vapor in the atmosphere can then condense to form clouds, and when these droplets become large enough, they fall as rain.
Learn more about evaporation from this link:
https://brainly.com/question/2013258
#SPJ11
Water vapor is formed through a process called evaporation.
When water is heated, the molecules gain energy and become more active. Some of these molecules gain enough energy to break free from the liquid and escape into the air as water vapor.
This process occurs naturally in bodies of water, such as oceans, lakes, and rivers, as well as on a smaller scale, like when water evaporates from a puddle or a wet surface.
Learn more about evaporation from this link:
https://brainly.com/question/2013258
#SPJ11
The attractive forces between sodium and chloride ions are called:
a)Bonding Forces
b)Gravity Forces
c)Pulse Forces
d)Interparticle Forces
Answers
Use the dropdown to create a unbalanced diagram with the dog using Applied Force pulling to the right.
Answers
State and explain modern periodic table. ⊕⊕
Answers
Explanation:
the physical and chemical properties of an element are periodic functions of their atomic number.
Answer:modern periodic table is published by Henry Moseley and he believes that ' properties of an elements are periodic function of the atomic number' and he classify the periodic table by groups and perieods so in the modern periodic table there are seven periods and eighteen groups.
if you have a 2.6 m solution of mgcl2 with a density of 1.17 kg l -1, what is the molar concentration of chloride ions in solution
Answers
The molar concentration of chloride ions in a 2.6 M solution of MgCl₂ can be calculated using the molar ratio of chloride ions to MgCl₂.
To find the molar concentration of chloride ions, we need to consider the molar ratio of chloride ions to MgCl₂ in the solution. MgCl₂ dissociates in water to form one Mg²⁺ ion and two Cl⁻ ions. This means that for every one mole of MgCl₂, we have two moles of chloride ions.
Given that the solution is 2.6 M, it means that there are 2.6 moles of MgCl₂ per liter of solution. Since the molar ratio of chloride ions to MgCl₂ is 2:1, we can multiply the molar concentration of MgCl₂ by the ratio to find the molar concentration of chloride ions.
Molar concentration of chloride ions = 2.6 M MgCl₂ × 2 = 5.2 M Cl⁻
Therefore, the molar concentration of chloride ions in the solution is 5.2 M.
It's important to note that the density of the solution is not directly used in this calculation, as the molar concentration is determined by the number of moles of solute (MgCl₂) in a given volume of solution, rather than its mass.
To know more about molar concentration refer here:
https://brainly.com/question/28270242#
#SPJ11
In covalent bonds, atoms give up electrons atoms accept electrons atoms share electrons electrons are always shared equally electrons cannot be shared equally
Answers
In chemistry, a covalent bond is an interatomic coupling formed when two atoms share an electron pair.
Thus, The electrical attraction of their nuclei for the identical electrons is what causes the binding. When the linked atoms' combined energies are lower than those of widely spaced atoms, a covalent connection is created.
The inorganic elements hydrogen, nitrogen, chlorine, water, and ammonia (H2, N2, Cl2, H2O, NH3) as well as all organic compounds are examples of molecules with covalent connections.
Covalent bonds have a direction, which means that the atoms they are bonded to favour particular alignments with one another. This results in the formation of specified forms for molecules, such as the angular (bent) structure of the H2O molecule.
Learn more about Covalent bonds, refer to the link:
https://brainly.com/question/10777799
#SPJ4
le chatlier'sprinciple states that if a system at equilirbium is disturbed, the equilibrium will shift to minimize the disturbance. true or false
Answers
The given statement, "le chatlier's principle states that if a system at equilibrium is disturbed, the equilibrium will shift to minimize the disturbance." is true because the system's natural tendency is to maintain a state of equilibrium.
Le Chatelier's principle states that if a system at equilibrium is subjected to a change in temperature, pressure, or concentration of reactants or products, the equilibrium will shift in such a way as to counteract the change and maintain equilibrium.
This principle is useful in predicting the direction in which a reaction will proceed when conditions are changed, and can also be used to optimize reaction conditions for maximum yield.
To know more about the le chatlier principle, here
brainly.com/question/9191469
#SPJ4
I need help w this pls

Answers
Answer:
70..... I think
Explanation:
Cuz if the bus travels 35 km every 30 mins this means that it travels 70 km per hour
what rule/principle states that electrons fill orbitals from lowest energy to highest enegery?
Answers
Answer:
The Aufbau Principle
Explanation:
In the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy level before occupying higher-energy levels.
Answer:
Aufbau principle
Explanation:
edge 2021
calculate the number of (a) nitrogen molecules (n2 molecules) and (b) nitrogen atoms (n atoms) in 0.253 g of nitrogen gas (n2)
Answers
The number of nitrogen molecules and nitrogen atoms in 0.253 g of nitrogen gas (N2) are as follows:a) Number of nitrogen molecules = 1.55 × 10²² N2 molecules
Number of nitrogen atoms = 3.1 × 10²² N atoms calculate the number of nitrogen molecules and nitrogen atoms In 0.253 g of nitrogen gas (N2), we use the following Firstly, we calculate the molar mass of nitrogen gas (N2).The molar mass of nitrogen gas (N2) is = 14 × 2 = 28 g/mol This means that one mole of nitrogen gas has a mass of 28 g. Next, we use the following formula to calculate the number of moles of nitrogen gas :N = m / MM where, N = Number of mole sm = Mass of the substance MM = Molar mass of the substance On substituting the values, we get:N = 0.253 g / 28 g/mol = 0.0090357 mol
Now, to calculate the number of nitrogen molecules and nitrogen atoms, we use the following formulas Number of nitrogen molecules = Avogadro's number × Number of moles of nitrogen gas Number of nitrogen atoms = 2 × Avogadro's number × Number of moles of nitrogen gas where, Avogadro's number = 6.022 × 10²³On substituting the values, we get Number of nitrogen molecules = 6.022 × 10²³ × 0.0090357 = 1.55 × 10²² N2 molecules) Number of nitrogen atoms = 2 × 6.022 × 10²³ × 0.0090357 = 3.1 × 10²² N atoms In summary, 0.253 g of nitrogen gas (N2) contains 1.55 × 10²² nitrogen molecules (N2 molecules) and 3.1 × 10²² nitrogen atoms (N atoms).
To know more about molecules Visit;
https://brainly.com/question/30465503
#SPJ11
Use The Periodic Table To Determine The Number Of 2p Electrons In F. Number Of 2p Electrons: Use The Periodic Table To Determine The Number Of 3p Electrons In Si, Number Of 3p Electrons: Use The Periodic Table To Determine The Number Of 3d Electrons In Fe. Number Of 3d Electrons: Use The Periodic Table To Determine The Number Of Ap Electrons In Kr. Number Of
Answers
Using the periodic table the number of electrons is determined as;
Fluorine (F) has 5 electrons in 2p orbital.
Silicon (Si) has 2 electrons in 3p orbital.
Iron (Fe) has 6 electrons in 3d orbital.
Krypton (Kr) has 6 electrons in 4p orbital.
The number of electrons in each orbital or the electronic configuration can be determined by the Aufbau principle, along with other principles such as the Pauli exclusion principle and Hund's rule.
1. The atomic number of Fluorine is 9, which means it has 9 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, and 5 electrons are in the 2p orbital. Therefore, the number of 2p electrons in F is 5.
2. The atomic number of Silicon is 14, which means it has 14 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, 6 electrons are in the 2p orbital, and 2 electrons are in the 3s orbital. Therefore, the number of 3p electrons in Si is 2.
3. The atomic number of Iron is 26, which means it has 26 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, 6 electrons are in the 2p orbital, 2 electrons are in the 3s orbital, and 6 electrons are in the 3p orbital. Therefore, the number of 3d electrons in Fe is 6.
4. The atomic number of Krypton is 36, which means it has 36 electrons. In the ground state, 2 electrons are in the 1s orbital, 2 electrons are in the 2s orbital, 6 electrons are in the 2p orbital, 2 electrons are in the 3s orbital, 6 electrons are in the 3p orbital, 10 electrons are in the 3d orbital, and 2 electrons are in the 4s orbital. Therefore, the number of 4p electrons in Kr is 6.
Learn more about electronic configuration: https://brainly.com/question/26084288
#SPJ11
The liquefied hydrogen halides have the normal boiling points given above. The relatively high boiling point of HF can be correctly explained by which of the following?
Answers
The relatively high boiling point of HF (hydrogen fluoride) compared to other hydrogen halides, it can be explained by the presence of hydrogen bonding.
Hydrogen bonding is a strong intermolecular force that occurs when a hydrogen atom is bonded to a highly electronegative element, such as fluorine.
In the case of HF, the difference in electronegativity between hydrogen and fluorine results in a polar bond, which allows for the formation of hydrogen bonds between neighboring HF molecules.
This bonding increases the energy required to separate the molecules, leading to a higher boiling point for HF compared to other hydrogen halides that do not experience such strong intermolecular forces.
For more information on high boiling point of halides refer https://brainly.com/question/40140
#SPJ11
Sucrose: density = 1.59 g/cm³. Your experiment showed a density of 1.68 g/cm³. What is the percent error *
Answers
Answer:
The answer is
5.66 %Explanation:
The percentage error of the density can be found by using the formula
\(Percentage \: \: error(\%) = \frac{error}{actual \: \: number} \times 100 \\ \)
From the question
actual density = 1.59 g/cm³
error = 1.68 - 1.59 = 0.09
The percentage error is
\(P(\%) = \frac{0.09}{1.59} \times 100\% \\ = 5.66037735...\)
We have the final answer as
5.66 %Hope this helps you
At what point in the cooking process is food typically seared?
Answers
Answer: when you look at the meat, and it looks beautiful like a crispy donut made by Gordan Ramsay
Explanation:
Hells Kitchen is kewl
Choose one astronomer from the chart to research. Create a poster/trifold board about the astronomer you chose. Make sure to include information about where and when they were born, what they did as astronomers, why what they researched mattered and how it affects how we view space and our planet today. Then, you will make a video of yourself as a living museum presentation. It should be at least 1 minute long, where you pretend to be that astronomer, and you will talk about yourself (the astronomer). Pay attention to the fact that the minimum length will only get you a 3 in the Content and Development area of the rubric. Most of your speech should be memorized, but you may use your poster and/or notecards to help guide you, if needed. You can email your video to your teacher or send a shareable link. Remember to read the rubric below so you can know how it will be graded. When you are ready, upload it replacing the “Lastname” in the saved document name with your last name. Be sure to include your sources in MLA formatting.
Answers
The planets in our solar system move in predictable patterns, as early astronomers found.
They were able to develop the rules of planetary motion and make precise predictions about the positions of the planets through careful observation and calculation. Later scientists might build on this work to grasp gravity, space, and the nature of our universe more fully. These discoveries are still being built upon today as we explore the solar system and beyond. It is useful to know how the planets move because it makes it easier to navigate satellites and spacecraft. In the end, the discoveries made by early astronomers have had a significant influence on how we perceive the universe and our role in it.
To know more about planetary motion, here
brainly.com/question/4978861
#SPJ1
--The complete Question is, What did early astronomers discover about the movements of the planets, and why does it matter to our understanding of space and our planet today? --
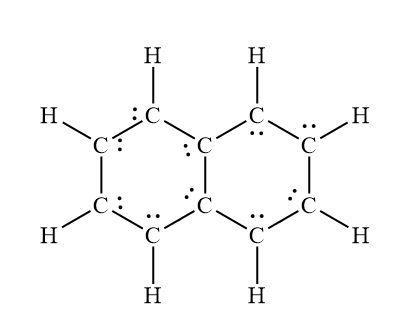
what is naphthalene lewis structure
Answers
Naphthalene is an organic compound with formula C ₁₀H ₈ whose molar mass is 128.1705 g/mol. The Lewis structure of the naphthalene is mentioned here.
What is Naphthalene?Naphthalene is the uncomplicated polycyclic aromatic hydrocarbon, and white crystalline solid with a property of atmosphere that is noticable at concentrations as low as 0.08 ppm by mass.
Naphthalene is construct from crude oil or coal tar. It is also construct when things burn, so naphthalene is found in cigarette smoke, car exhaust, and smoke from forest fires. Naphthalene is also used as a pest repellent and insecticide. Naphthalene was first record as a pesticide in the United States in 1948.
Learn more about the Naphthalene here: https://brainly.com/question/1387132
Which of the following is a liquid?
milk
oxygen
cheese
Answers
Briefly explain how a barometer is used to
measure atmospheric pressure?
Answers
Answer:
A mercury barometer is an instrument used to measure atmospheric pressure in a certain location and has a vertical glass tube closed at the top sitting in an open mercury-filled basin at the bottom. Mercury in the tube adjusts until the weight of it balances the atmospheric force exerted on the reservoir.
What is chemistry? How is it used in your everyday life? Give at least two examples and explain your answers. Must have at least 4 sentences to get full credit.
Answers
I am playing with my
Answers
Answer:
wow ok have fun
Explanation:
Answer:
hiiiiiiiii
Explanation:
thank you
What is commensalism? Give two examples.
Answers
Answer:
Commensalism is a relationship between two organisms in which one organism benefits, and the other one is left unaffected.
Examples:
1. Barnacles on a sea turtle
2. Tree frogs and plants (they use them as protection)
1. Barnacles on a sea turtle
2. Tree frogs and plants (they use them as protection)
hope this helps
True or False: Mitochondrial ATP synthase can synthesize ATP after it is extracted from broken mitochondria
Answers
False. Mitochondrial ATP synthase is a protein complex located in the inner mitochondrial membrane that is responsible for synthesizing ATP from ADP and inorganic phosphate during oxidative phosphorylation.
It is an integral part of the electron transport chain, which is the process by which cells generate most of their ATP. The ATP synthase complex requires a proton gradient across the inner mitochondrial membrane to function properly. When mitochondria are broken or disrupted, the proton gradient is lost, and ATP synthesis ceases.
Therefore, ATP synthase cannot synthesize ATP once it is extracted from broken mitochondria. In fact, ATP synthase is highly sensitive to disruption and is often used as a marker for mitochondrial damage or dysfunction. This is why it is crucial to handle mitochondria carefully during experiments to ensure that they remain intact and functional. Overall, mitochondrial ATP synthase is a critical component of cellular energy metabolism and plays an essential role in maintaining proper cellular function.
To know more about ATP synthase
https://brainly.com/question/893601
#SPJ11
in a hotel breakfast bar you see an older woman sticking a fork into a toaster to remove a piece of toast. You wamn her not to do that and she says. "You know, I always
wondered why fortes conduct electricity so well" As an astute chemistry student how would you explain it to her? Make sure you provide a relationship to
electronegativity proton pull and electron distribution in your answer
Answers
Answer: I guess have to like explain to her why to not stick the fork into the toaster.
Explanation:
Hope this helps
Answer:
its just how would you explain how it happens how the electricity passes through the fork but in a easy understandable way
Explanation:
for example... dont do that because......................then will go through the fork then to your arm and will cause....................... then you die
what kind of element is ca main group elements droppable transition metals droppable inner transition metals
Answers
The element Ca, calcium is the main group element. The Ca belongs to the group 2 and the period 4.
The Ca is the main group element , belongs to the period 4 and the group 2 of the periodic table. The calcium belongs to the s-block element. The s block and p block elements are the main group element.
The s-block elements are separated into the two groups: the Group 1 is the alkali metals and Group 2 is the alkaline earth metals. The Groups 13 - 18 belongs to the p-block elements which are basic metals, the metalloids, the nonmetals, the halogens, and the noble gases.
To learn more about main group here
https://brainly.com/question/14818173
#SPJ4