what is the ph of a 0.040 m ca(oh)2 solution?
Answers
To find the pH of a given Ca(OH)₂ solution, you need to first determine the concentration of OH⁻ ions produced by the dissociation of Ca(OH)₂. Then, calculate the pOH by taking the negative logarithm of the OH⁻ concentration. Finally, find the pH by subtracting the calculated pOH from 14 which is pH ≈ 12.9
To find the pH of a 0.040 M Ca(OH)₂ solution, follow these steps:
Step 1: Identify the dissociation of the compound
Ca(OH)₂ dissociates into ions in water as follows:
Ca(OH)₂ → Ca²⁺ + 2OH⁻
Step 2: Determine the concentration of hydroxide ions (OH⁻)
For every mole of Ca(OH)₂, there are 2 moles of OH⁻ ions produced. Therefore, you can calculate the concentration of OH⁻ ions by multiplying the given molarity of Ca(OH)₂ by 2:
0.040 M * 2 = 0.080 M OH⁻
Step 3: Calculate the pOH
The pOH is the negative logarithm of the hydroxide ion concentration:
pOH = -log[OH⁻] = -log(0.080)
Use a calculator to find the logarithm:
pOH ≈ 1.1
Step 4: Calculate the pH
To determine the pH, you need to use the relationship between pH and pOH, which is as follows:
pH + pOH = 14
Now, substitute the calculated pOH value into the equation and solve for the pH:
pH = 14 - pOH = 14 - 1.1
pH ≈ 12.9
So, the pH of a 0.040 M Ca(OH)₂ solution is approximately 12.9. This indicates that the solution is alkaline, as the pH value is greater than 7.
In summary, to find the pH of a given Ca(OH)₂ solution, you need to first determine the concentration of OH⁻ ions produced by the dissociation of Ca(OH)₂. Then, calculate the pOH by taking the negative logarithm of the OH⁻ concentration. Finally, find the pH by subtracting the calculated pOH from 14.
To know more about pH refer here: https://brainly.com/question/31132561#
#SPJ11
Related Questions
What is the pH of a buffer solution prepared by mixing 20.0 mL of 0.0800 molL−1NaOH with 20.0 mL of 0.130 molL−1 cacodylic acid?
Answers
Answer:
pH = 6.20
Explanation:
The pKa of cacodylic acid is 6.
To solve this question we must use Henderson-Hasselbalch equation:
pH = pKa +log [A⁻] / [HA]
Where pKa is the pKa of the weak acid = 6
And [] could be taken as the moles of A⁻ the conjugate base, and HA, the weak acid.
The moles of the NaOH added to the solution of the weak acid are = Moles A⁻
And moles HA = Initial moles HA - Moles NaOH added
Initial moles HA:
0.0200L * (0.130mol / L) = 0.00260 moles
Moles NaOH:
0.0200L * (0.0800mol / L) = 0.00160 moles = [A⁻]
Moles HA =
0.00260 moles - 0.00160 moles = 0.00100 moles = [HA]
pH = 6 +log [0.00160 moles] / [0.00100 moles]
pH = 6.20The pH of the resulting solution is 1.6.
Let cacodylic acid be HA, mixing cacodylic acid and NaOH, the following occurs;
HA(aq) + NaOH(aq) ------> NaA(aq) + H2O(l)
Number of moles of NaOH = 0.0800 molL−1 × 20.0/1000 = 0.0016 moles
Number of moles of HA = 20.0/1000 × 0.130 = 0.0026 moles
We can see that the HA is in excess, Number of moles of excess acid =
0.0026 - 0.0016 = 0.001 moles
Total volume of solution = 20.0 mL + 20.0 mL = 40 mL or 0.004 L
Molarity of excess acid = 0.001 moles/0.004 L = 0.025 M
pH = -log[H^+]
pH = -log[0.025 M]
pH = 1.6
Learn more: https://brainly.com/question/2510654
Place the following compounds in order of increasing magnitude of lattice energy. Na2o nacl mgo kbr.
Answers
KBr < NaCl < MgO <Na2O
KBr < NaCl>The formulation is MgO. Magnesium oxide could have the most important lattice power as it has the most important appeal among the 2 ions.
Mg2+ ion has a smaller length whilst in comparison to Ca2+ advert thus MgO has the very best lattice power some of the given compounds.
Li¹⁺ F¹⁻
K¹⁺ Br¹⁻
Na¹⁺ Cl¹⁻
Because the compounds all have the identical fee we want to have a take a observe their role at the periodic table.
KBr < NaCl < MgO <Na2O
K is the bottom ion withinside the group, then comes Na, and at the very best is Li.
Learn more about lattice energy here https://brainly.com/question/13169815
#SPJ4
solubility rules and net ionic equations pogil answer key
Answers
The sort of ions in a salt determines the solubility of a compound. While certain salts dissolve in water, some do not. When two soluble salts are combined in water, a third insoluble salt may result from the mixing.
The interactions between two substances that are dissolved in water are depicted using net ionic equations. The outcomes of a twofold displacement (replacement) reaction will be accurately predicted. An ion that is present during a reaction but does not participate in it is referred to as a spectator ion. A net ionic equation only displays the ions that change as a result of a chemical reaction. (Net ionic equations do not include spectator ions.)
To learn more about solubility click here https://brainly.com/question/29857840
#SPJ4
complete question: Using Solubility Rules to predict the formation of a precipitate. Write molecular equations and net ionic equations.
Calculate the pH of 0.10 M solution of NaBO2.
a. 9.84
b. 12.89
c. 10.48
d. 11.11
e. 2.89
Answers
The correct answer is option c. 10.48. To calculate the pH of a solution of NaBO2, we need to first identify the relevant chemical reaction. NaBO2 is a salt of a weak base (BO2-) and a strong acid (Na+). When it dissolves in water, it undergoes hydrolysis, meaning it reacts with water to form its conjugate acid and base:
NaBO2 + H2O ⇌ BO2- + Na+ + H2O
The equilibrium constant for this reaction is called the base dissociation constant (Kb). To find the pH of the solution, we need to use the Kb value of BO2- and the concentration of NaBO2 to calculate the concentration of OH- ions in the solution. The Kb value of BO2- is 5.6 x 10^-10. Using the concentration of NaBO2 (0.10 M), we can calculate the concentration of BO2- in the solution. Since NaBO2 is a strong electrolyte, it completely dissociates into its ions, so the concentration of Na+ can be ignored in this case: [BO2-] = [OH-] = Kb * [NaBO2] / [H2O]
Substituting the values, we get: [BO2-] = [OH-] = 5.6 x 10^-10 * 0.10 / 1.0 = 5.6 x 10^-11 M
Now, to find the pH, we need to calculate the pOH first:
pOH = -log[OH-] = -log(5.6 x 10^-11) = 10.25
pH + pOH = 14 (at 25°C)
pH = 14 - 10.25 = 3.75
To know more about pH
https://brainly.com/question/172153
#SPJ11
In what ways are the electronic structures of the group 14 (4A) elements similar? In what ways are they different?
Answers
Answer:
The electron structures of the group 14 (4A) elements are similar in that they are all part of the carbon group. Thus, they each have 4 electrons in their valence shell. Also, most of these elements are found in our daily lives except germanium. They are different in that their properties differ greatly despite them being somewhat alike.
Explanation:
I hope this helps! I took this class last year.
Would you expect corrosion to occur more rapidly in a desert or in a rainforest
Answers
Corrosion is the process of deterioration of metals or materials that are exposed to environmental conditions. The rate of corrosion is affected by several factors such as humidity, temperature, and pH.
In a desert, the air is dry, and there is low humidity, which makes the environment less corrosive than in a rainforest. On the other hand, a rainforest is a highly humid environment with a lot of moisture in the air. The moisture, together with oxygen, accelerates the corrosion process of metals.
In summary, the rate of corrosion of metals is influenced by a variety of factors, including the humidity, temperature, and pH of the environment. Since a rainforest is highly humid, the rate of corrosion is expected to be faster in a rainforest than in a desert. Therefore, if metals are exposed to the two environments, the ones exposed to the rainforest are more likely to corrode faster than those exposed to the desert environment.
To know more about temperature visit:
https://brainly.com/question/15520591
#SPJ11
Octane has the following chemical equation. C8H18
B.1
How many atoms of Carbon C are in 3 molecules of Octane 3C8H18
B.2
How many atoms of Hydrogen H are in 2 molecules of Octane 2C8H18
Answers
Answer:
36 atoms
Explanation:
2C8H18
meaning
2 multiples 18 giving 36atoms.
Answer:
B1: 24
Explanation:
3 times 8 equals 24 atoms.
Ga X ha the molecular formula C5Hx. A ma of 1. 44g of X occupie a volume of 0. 32 dm3
at 2. 0 atm and
400 K. Find the value of x
[H = 1; C = 12; R= 0. 08 atm. Dm3. K-1. Mol-1]
Answers
The molecular formula for C5Hx in this question is C5H12
In this case, molecular formula can be determined by calculated the molecular weight relative for each molecule. The molecular weight relative for each molecule calculated by using ideal gas equation:
PV=mRT/Mr
with,
P = Pressure of the system
V = Volume occupied by gas
m = mass of C5Hx
R = ideal gas constant
T = Temperature of the system
Mr = molecular weight of C5Hx
2 x 0.32 = 1.44 x 0.08 x 400 /Mr
Mr = 1.44 x 0.08 x 400/ (2 x 0.32)
Mr = 72
Mr of C5Hx = 6 x 12 + 1 x X
72 = 60 + X
X = 12
C5Hx = C5H12
Learn more about molecular formula here: https://brainly.com/question/1247523
#SPJ4
from the gc data, assign the signals to 2-methyl-2-butene and 2-methyl-1-butene. how were the assignments made?
Answers
A GC-MS produces muti data, including chromatograms for quantitative and qualitative analysis, mass spectra for confirming identity, identifying unknown analytes, and determining the structural and chemical properties of molecules.
What do you discover with GC-MS data?The GCMS uses a standard, or a proportion of the component that has already been established and has also been measured on the GCMS, to quantify the concentration of each chemical in a sample.
On a chart known as a chromatogram, a peak for each substance that the GC separates is used to symbolize it. The number of peaks reflects the sample's concentration of separated components. Each peak's location reveals the retention time of the compound.
To know more about compounds visit :
https://brainly.com/question/14117795
#SPJ4
When sugar is added to a sugar solution, the sugar does not dissolve. Which
term describes the original sugar solution?
O A. Semisaturated
O B. Supersaturated
C. Saturated
D. Unsaturated
Answers
Answer:Supersaturated
Explanation:The answer is supersaturated because supersaturation is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. It is supersaturated because there is already a sugar solution and adding another sugar is more than the maximum amount of solute.
What would happen if the mechanisms for communication of ideas and discussion of science production did not exist?
Please make a response of 5 complete lines
Answers
The thing that will happen if the mechanisms for communication of ideas and discussion of science production did not exist is that researches will be harder to make.
What is communication?The transmission of information is commonly defined as communication. The term can also refer to the message itself or to the field of study that investigates such transmissions.
Because the active role of all participants in this process is recognized, the term "science communication" is now more commonly used than "popularization." Making science more accessible to the general public can help to alleviate society's current confusion and instill hope for the future.
Effective communication is critical to addressing the diversity and inclusion issues that plague science. Without effective communication, gatekeeping will persist, and barriers to understanding science will remain.
Aside from benefiting society as a whole, communicating outside the scientific community can help a researcher's career by increasing the impact of their latest findings, fostering new collaborations across sectors, raising their public profile, and opening doors to unexpected opportunities
Learn more about communication on:
https://brainly.com/question/26152499
#SPJ1
which of the following pairs of substances would make the best buffer with a basic ph? ka for hc3h2o2
Answers
To determine the best buffer with a basic pH using the given pKa value for HC3H2O2, we need to find a pair of substances where one acts as a weak acid (HC3H2O2) and the other as its conjugate base (C3H2O2-).
The pKa of HC3H2O2 represents the pH at which the acid is 50% ionized. Since we want a basic pH, we need a pKa value that is slightly higher than the desired pH. Let's assume the desired pH is around 9.
A quick calculation shows that a pKa of 8.5 would be suitable for our purpose.
Now, we need to find a conjugate base with a pKa close to 8.5. One example is ammonium acetate (NH4C2H3O2) with a pKa of 9.25. When ammonium acetate is dissolved in water, it dissociates into NH4+ (conjugate acid) and C2H3O2- (conjugate base).
Therefore, the best buffer pair for a basic pH would be HC3H2O2 (acetic acid) and NH4C2H3O2 (ammonium acetate).
The pKa value of HC3H2O2 is not provided in the question. However, assuming we have the pKa value of HC3H2O2, we can use it to calculate the pH range over which the buffer will be effective.
The Henderson-Hasselbalch equation is commonly used to calculate the pH of a buffer solution:
pH = pKa + log ([A-]/[HA])
In this equation, [A-] represents the concentration of the conjugate base, and [HA] represents the concentration of the weak acid.
To create a buffer with a basic pH, we need a pKa slightly higher than the desired pH. Assuming a desired pH of 9, we can use a pKa value around 8.5.
Let's consider ammonium acetate (NH4C2H3O2) as a potential conjugate base for HC3H2O2. The pKa value of ammonium acetate is 9.25.
Using the Henderson-Hasselbalch equation, we can determine the pH range over which the buffer will be effective. For a basic pH, we want the [A-]/[HA] ratio to be high, indicating a significant concentration of the conjugate base.
With a pKa of 8.5 for HC3H2O2 and a pKa of 9.25 for NH4C2H3O2, we can calculate the pH range as follows:
pH = pKa + log ([A-]/[HA])
pH = 8.5 + log ([C2H3O2-]/[HC3H2O2])
To ensure a high [C2H3O2-]/[HC3H2O2] ratio, we can adjust the concentrations of the weak acid and its conjugate base accordingly. By choosing appropriate concentrations, we can achieve a pH in the desired range.
Based on the given pKa value for HC3H2O2, the best buffer pair for a basic pH would be HC3H2O2 (acetic acid) and NH4C2H3O2 (ammonium acetate) with a pKa of 8.5 for HC3H2O2 and a pKa of 9.25 for NH4C2H3O2. By adjusting the concentrations of the weak acid and its conjugate base
To know more about best buffer and basic pH, Visit,
brainly.com/question/24262133
#SPJ11
A gas occupies a volume of 10 liters at a pressure of 0.5 atm. What is the pressure if the volume increases to 25.0 L? *
A.0.4 atm
B.0.3 atm
C.0.2 atm
D.0.1 atm
Answers
Answer:
0.2atm
Explanation:
By Boyle's law
P1V1=P2V2
0.5 x 10 = P2 x 25
therefore, P2= 0.2atm
is sio2 most likely a molecular, metallic, ionic, or covalent-network solid?
Answers
Sio2, also known as silicon dioxide or silica, is most likely a covalent-network solid.
To determine the type of solid, we need to analyze the nature of bonding in SiO2. Silicon (Si) and oxygen (O) are both nonmetals, and their bonding is typically covalent.
In SiO2, silicon forms four covalent bonds with four oxygen atoms, and each oxygen atom forms two covalent bonds with two silicon atoms. This results in a three-dimensional network structure of alternating silicon and oxygen atoms.
Covalent-network solids have a high melting point, are hard and brittle, and do not conduct electricity because the electrons are localized within the covalent bonds. In the case of SiO2, the strong covalent bonds between silicon and oxygen atoms give rise to its characteristic properties.
Based on the nature of bonding in SiO2, it is most likely a covalent-network solid. The three-dimensional network structure formed by covalent bonds between silicon and oxygen atoms is responsible for its high melting point and other properties associated with covalent-network solids.
To know more about covalent-network visit:
https://brainly.com/question/30037205
#SPJ11
Which quantum number represents the individual orbital (orientation of the orbital) an electron occupies?
Answers
Magnetic Quantum Number represents the individual orbital (orientation of the orbital) an electron occupies.
What exactly is the magnetic quantum number?Between spin and azimuthal quantum number, the magnetic quantum figure comes in third on the list. The electron is placed in one of the numerous orbitals established by the partition of the subshells (such as s, p, d, and f). It determines the spatial direction of an orbital of a certain energy (n) and form (I).
Which four quantum numbers are there?The primary quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number are the four quantum numbers that comprise an atom (ms)
Learn more about quantum number here:
brainly.com/question/24204727
#SPJ4
Calcium hydroxide and phosphoric acid react to form calcium phosphate and
water
Answers
Answer:
if you are asking for the chemical reaction:
Ca(OH)2 + H3PO4-------> Ca3(PO4)2 + H2O
Explanation:
In this reaction, phosphoric acid, H3PO4 , a weak acid, will react with calcium hydroxide, Ca(OH)2 , a strong base, to produce calcium phosphate, Ca3(PO4)2 , an insoluble salt, and water.
is the reaction shown here esterification, hydrogenation, hydrolysis, saponification, or substitution?
Answers
However, a brief description of each term esterification, hydrogenation, hydrolysis, saponification, or substitution is
1. Esterification: A reaction between an acid and an alcohol to form an ester and water.
2. Hydrogenation: A reaction where hydrogen is added to a molecule, typically involving the reduction of double or triple bonds in an unsaturated compound.
3. Hydrolysis: A reaction involving the breakdown of a compound by adding water.
4. Saponification: A process in which a fat or oil reacts with an alkali to produce soap and glycerol.
5. Substitution: A reaction in which an atom or a group of atoms in a molecule is replaced by another atom or group of atoms.
To know more about esterification visit:
https://brainly.com/question/16010744
#SPJ11
Please the app isn’t working and I can’t find other questions that got answered

Answers
Given:
Sum of masses of two isotopes = 371.9087 u
Re-185 natural abudance = 37.40%
Re-187 natural abudance = 62.60%
Known:
atomic weight of Re = 186.207 u
Atomic mass of Re-185:
To find the atomic mass of Re-185, take the total mass given and subtract atomic weight.
abundance of Re-185 = 37.40% = 0.3740
(371.9087 - x) = atomic weight of Re-187 in u
To find mass of Re-187:
abundance of Re-187 = 62.60% = 0.6260
Solution:
Step 1. Multiply x times the abundance of Re-185 and multiply (371.9087 - x) times the abundance of Re-187.
Re-185: (0.3740)(x) = 0.3740x
Re-187: (0.6260)(371.9087 - x) = 232.8148462 - 0.6260x
Step 2. Add the results and set them equal to 186.207.
0.3740x + 232.8148462 - 0.6260x = 186.207
Step 3. Solve for x by subtracting 232.8148462 from both sides and then divide both sides by -0.2520.
0.3740x + 232.8148462 - 0.6260x - 232.8148462 = 186.207 -
232.8148462
0.3740x - 0.6260x = -46.6078462
-0.2520x = -46.6078462
-0.2520x/-0.2520x = -46.6078462/-0.2520
x = 184.9517706 u
Step 4. Atomic weights of Re-185 and Re-187.
x = 185.0 u = the atomic weight of Re-185
(371.9087 - 184.9517706) = 186.9569294 = 187.0 u = the atomic weight of Re-187
Therefore the atomic weight of Re-185 is 185.0 u, and the atomic weight of Re-187 is 187.0 u.
Natural Causes of Climate Change:
Pick one cause of natural climate change. Explain what it is and how
it causes dimate change. What is the effect of the climate change?
Helpp plzzz

Answers
Answer:
cause of climate change: The earth's climate is affected and changed through a lot of natural causes like volcanic eruptions.
how it causes change: Volcanoes can be extremely dangerous to earth because it can impact climate change. During major explosive eruptions huge amounts of volcanic gas, aerosol droplets, and ash are injected into the stratosphere.
what is the effect of the change: Major eruptions alter the Earth's radiative balance because volcanic aerosol clouds absorb terrestrial radiation, and scatter a significant amount of the incoming solar radiation, an effect known as "radiative forcing" that can last from two to three years following a volcanic eruption.
i hope you get it correct!! <3 can i plz get a brainlest!
the chemical reaction that generated tall of the heat in the stove was caused by the combustion of methane (CH4). this reaction raleases 808kj of energy per mole of methane. if you turned your stove on for 5 minutes an used up 38.0 (27g) of methane, how much energy did you release?
Answers
The amount of energy released would be 1917.96 kJ.
Energy released by combustionWe can start by calculating the number of moles of methane used up in the reaction:
Number of moles = mass / molar mass
molar mass of CH4 = 12.01 + 4(1.01) = 16.05 g/mol
Number of moles = 38.0 g / 16.05 g/mol = 2.37 mol (rounded to two decimal places)
Next, we can calculate the total energy released by the combustion of this amount of methane:
Energy released = energy released per mole x number of moles
Energy released = 808 kJ/mol x 2.37 mol = 1917.96 kJ (rounded to two decimal places)
Therefore, if you used up 38.0 g (or 2.37 mol) of methane in 5 minutes, you would have released approximately 1917.96 kJ of energy.
More on heat of combustion can be found here: https://brainly.com/question/14317568
#SPJ1
what role does lactase play in breaking apart the disaccharide lactose?
lactase provides a binding site for lactose to initiate chemical breakdown.
lactase lowers the activation energy needed to begin breaking down lactose.
lactase releases heat during the breakdown of lactose.
lactase prevents too many disaccharide molecules from clumping together during chemical reactions.
Answers
Role does lactase play in breaking apart the disaccharide lactose is lactase prevents too many disaccharide molecules from clumping together during chemical reactions
Lactose is a disaccharide consisting of 2 monosaccharides, glucose and galactose, linked together via a β-1→4 bond and hydrolysis of this bond requires a specific enzyme called lactase which digests lactose to its components allowing the uptake of glucose and galactose from the intestine
When we eat something containing lactose, an enzyme in the small intestine called lactase breaks it down into simpler sugar forms called glucose and galactose and this glucose and galactose are disaccharide and these simple sugars are then absorbed into the bloodstream and turned into energy
Know more about lactase
https://brainly.com/question/28754200
#SPJ1
What volume of a 2. 000 m hcl solution is needed to generate 100. 0 ml of a 0. 1000 m solution of hcl?.
Answers
5ml of HCl is needed to convert a 2M molarity solution into a 0.1M solution of HCl that is 100ml in volume.
The HCl solution has a fixed molarity of 2M, and 100ml of a 0.1M molecular-weight solution is required for preparation. It is now necessary to determine the volume that will be required to make the 100ml 0.1M solutions.
M₁V₁ = M₂V₂
It is the dilution formula that should be used to achieve this.
We obtain,
2 x V₁ = 0.1 x 100 by entering the supplied values into the dilution formula.
2V1 = 10 V1
V₁ = 10/2
V₁ = 5ml
As a result, 5ml of HCl is needed to make 100ml of a 0.1M solution.
To know more about volumetric analysis, click below:
https://brainly.com/question/15077942
#SPJ4
HELP MEEE PLEASEEEEEEEEEEEEEE
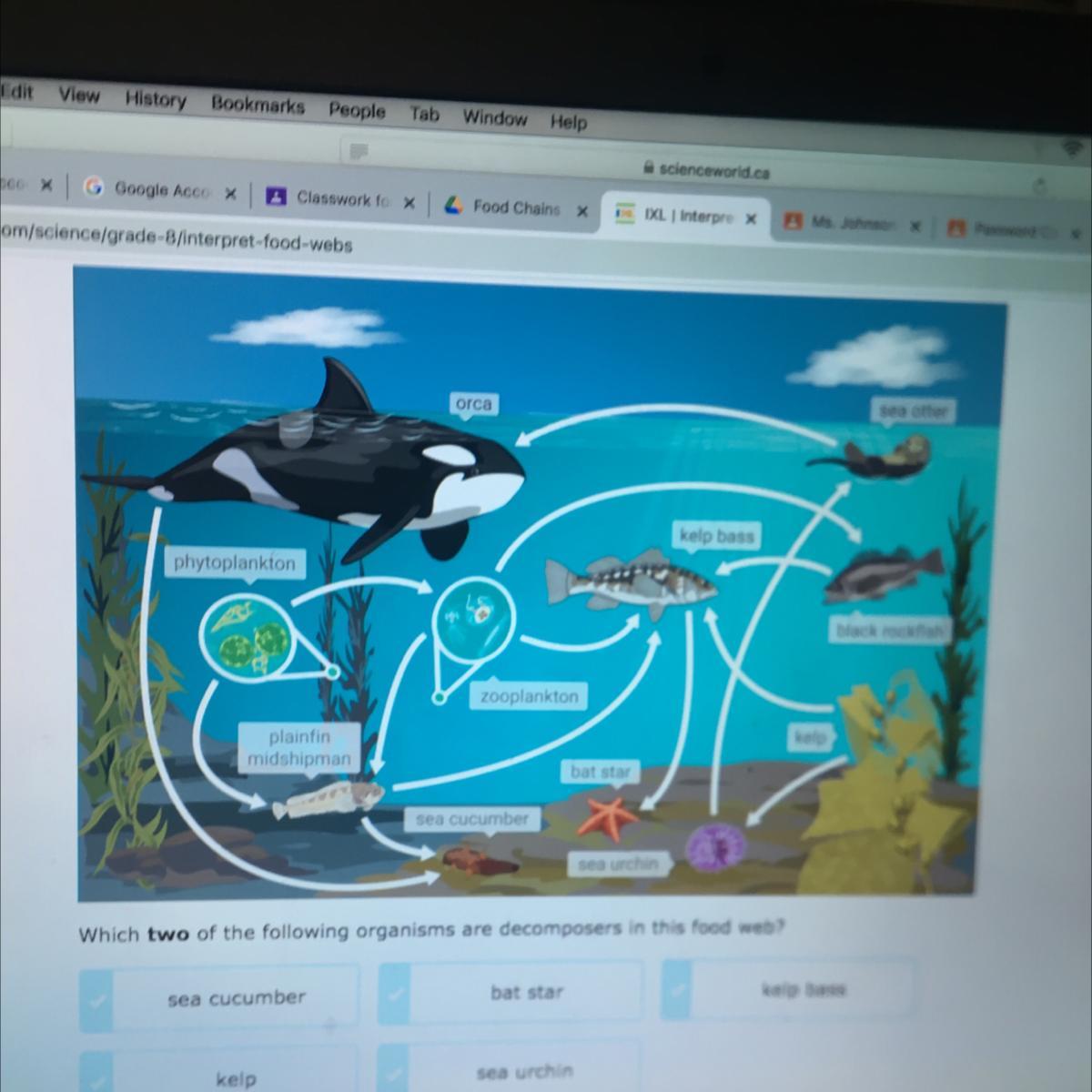
Answers
I think its the sea cucumber and sea urchin
but it could be the star too
water is added to a 8.23 g sample of tacl5. the only products are 5.71g of a solid containing only tantalum, chlorine and oxygen and 3.35 g of a gas which is 97.2% chlorine and the remainder is hydrogen. (a) determine the empirical formula of the gas. (b) what fraction of the chlorine of the original compound is in the solid? (c) determine the empirical formula for the solid produced. (d) write a balanced equation for the reaction between tantalum pentachloride and water
Answers
The empirical formula is the simplest formula for a compound which is defined as the ratio of subscripts of smallest possible whole number of the elements present in the formula. It is also known as the simplest formula.
write a balanced equation for the reaction between tantalum pentachloride and water?
Tantalum Pentachloride is used as the chlorinating agent of the organic compound, chemical intermediates, and preparation as tantalum.TaCl5 is used in the preparation of catalyst for the polycyclotrimerizations of alkenediynes, chloro-aryloxide compounds and for the plasma-enhanced atomic layer deposition of tantalum nitride films. This product is involved in the preparation of tantalum(V) oxychloride.Tantalum oxide (Ta2O5) is one of the most important transition metal oxides because of its extraordinary physical and chemical properties, including high dielectric and refractive coefficients and excellent photoelectric performance.To learn more about chlorine refers to:
https://brainly.com/question/29794366
#SPJ4
How can you tell the difference between two clear liquids
Answers
Answer:
To identify a pure liquid substance using the physical properties of solubility, density, and boiling point. The physical properties of a pure substance can be measured without changing the composition of the substance.
Explanation:
rank the nitrogen‑containing aromatic molecules in order of increasing basicity. you are currently in a ranking module. turn off browse mode or quick nav, tab to move, space or enter to pick up, tab to move items between bins, arrow keys to change the order of items, space or enter to drop. least basic most basic
Answers
The order of increasing basicity is: Aniline < Pyridine < Pyrrole < Ammonia.
To rank the nitrogen-containing aromatic molecules in order of increasing basicity, we need to consider the electron-donating ability of each molecule's nitrogen atom. The more electron-donating the nitrogen atom, the more basic the molecule.
1. Aniline (\(C_6H_5NH_2\)): Aniline is the least basic molecule among the given options. The nitrogen atom in aniline is directly attached to an aromatic ring, which has a partial negative charge. This partial negative charge reduces the electron-donating ability of the nitrogen atom, making it less basic.
2. Pyridine (\(C_5H_5N\)): Pyridine is more basic than aniline. The nitrogen atom in pyridine is also attached to an aromatic ring, but the nitrogen atom in pyridine is less affected by the partial negative charge of the ring. As a result, the nitrogen atom in pyridine can donate electrons more easily, making it more basic than aniline.
3. Pyrrole (\(C_4H_5N\)): Pyrrole is more basic than pyridine. The nitrogen atom in pyrrole is directly involved in a conjugated pi-system, which provides additional electron density to the nitrogen atom. This increased electron density allows the nitrogen atom in pyrrole to donate electrons more readily, making it more basic than pyridine.
4. Ammonia (\(NH_3\)): Ammonia is the most basic molecule among the given options. Unlike the previous three molecules, ammonia is not aromatic. However, it is still a nitrogen-containing compound. The lone pair of electrons on the nitrogen atom in ammonia is not involved in any aromatic or conjugated pi-system, making it highly available for donation. This makes ammonia the most basic among the given molecules.
For more such question on basicity visit:
https://brainly.com/question/172153
#SPJ8
In the Bohr model, ____ electrons fill the first energy level, ____ electrons fill the second energy level, and ___ electrons fill the third energy level.
A) 1, 2, 3
B) 2, 8, 8
C) 8, 8, 8
D) 2, 8, 18
Answers
What geological process changes pieces of rocks, minerals, and other material into sedimentary rock?
Answers
Helpppp!!!^_________^

Answers
Answer:
Standard boiling point
Explanation:
Note that there are 2 major units of pressure except Pa .
baratmAt 1 atm pressure the boiling temperature is called normal boiling point.
At 1 bar pressure the boiling temperature is called standard boiling point
Determine the number of grams of carbon dioxide that can be formed from 0.500 grams of iron oxide and an excess of carbon.
Answers
Taking into account the reaction stoichiometry, 0.2066 grams of CO₂ are formed from 0.500 grams of iron oxide and an excess of carbon.
Reaction stoichiometryIn first place, the balanced reaction is:
2 Fe₂O₃ + 3 C → 4 Fe + 3 CO₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Fe₂O₃: 2 moles C: 3 molesFe:4 moles CO₂: 3 molesThe molar mass of the compounds is:
Fe₂O₃: 159.7 g/moleC: 12 g/moleFe: 55.85 g/moleCO₂: 44 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Fe₂O₃: 2 moles ×159.7 g/mole= 319.4 gramsC: 3 moles ×12 g/mole= 36 gramsFe: 4 moles ×55.85 g/mole= 223.4 gramsCO₂: 3 moles ×44 g/mole= 132 gramsMass of CO₂ formedThe following rule of three can be applied: if by reaction stoichiometry 319.4 grams of Fe₂O₃ form 132 grams of CO₂, 0.500 grams of Fe₂O₃ form how much mass of CO₂?
\(mass of CO_{2} =\frac{0.500 grams of Fe_{2}O_{3}x132 grams of CO_{2} }{319.4 grams of Fe_{2}O_{3}}\)
mass of CO₂= 0.2066 grams
Then, 0.2066 grams of CO₂ are formed from 0.500 grams of iron oxide and an excess of carbon.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1